Figures & data
Figure 1. Transmission electron microscopy of seminal prostasomes from normozoospermic men separated by lectin-affinity chromatography. Micrographs of vesicles (70–170 nm) in a concanavalin A (Con A)-non-bound fraction (A). A dense vesicle (thin arrow), a dark vesicle (thick arrow), a light vesicle (white arrow), and amorphous material (arrowhead) can be clearly observed. Single (50–90 nm) or aggregated vesicles were present in a Con A-bound fraction (B). The insert shows the typical cup-shape appearance enlarged. Micrographs of vesicles (50–100 nm) in wheat germ agglutinin (WGA)-non-bound fraction (D) and vesicles (50–100 nm) in a WGA-bound fraction (E). Vesicles in the high-pH-eluted fraction from Con A column (C) and low-pH-eluted fraction from WGA column (F) were scarce.
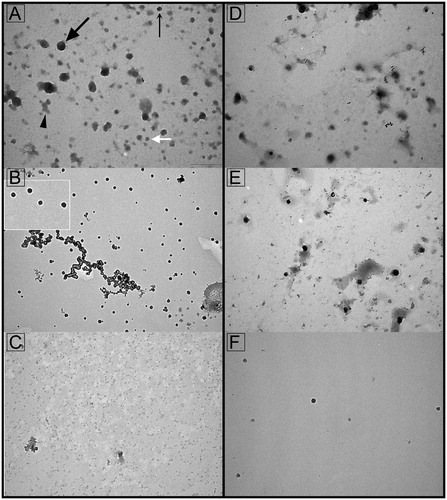
Figure 2. Protein composition of seminal prostasomes from normozoospermic men separated by lectin-affinity chromatography. Seminal prostasomes separated by lectin-affinity chromatography were resolved on 10% SDS-PAGE under reducing and denaturing conditions and stained with silver. Representative total protein patterns of non-bound (nb) and bound (b) fractions separated on a Con A column and on a WGA column were shown. Samples were loaded as isolated (equal volume/line to reflect yield), and total protein amounts were: line 1 (32 μg), line 2 (9 μg), line 3 (17 μg), line 4 (11 μg), line 5 (18 μg), line 6 (5 μg), line 7 (14 μg), line 8 (12 μg). Characteristic prostasome-associated bands in the region of 90–150 kDa are marked (asterisk). Numbers indicate the position of molecular mass standards (kDa). sPro-N1 and sPro-N2: different isolates of seminal prostasomes from normozoospermic men.
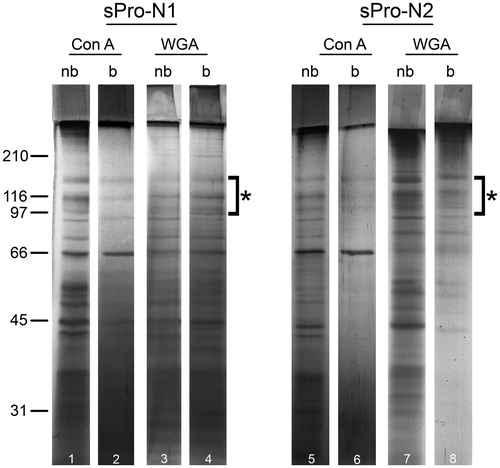
Table 1. Surface-associated markers of seminal prostasomes from normozoospermic men separated by lectin-affinity chromatography.
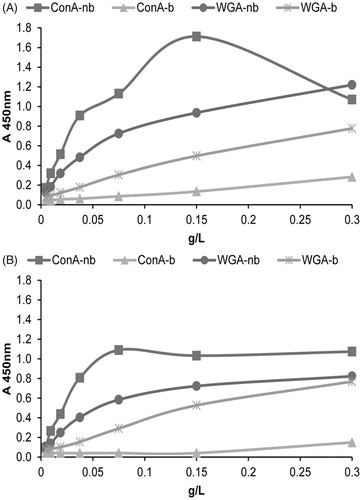
Figure 3. Surface glycosylation of seminal prostasomes from normozoospermic men separated by lectin-affinity chromatography (LAC). LAC-separated fractions of seminal prostasomes from normozoospermic men: Con A-non-bound (Con A-nb), Con A-bound (Con A-b), WGA-non-bound (WGA-nb), and WGA-bound (WGA-b) fractions were immobilized and re-probed with Con A (A) and WGA (B) by solid-phase binding assay.
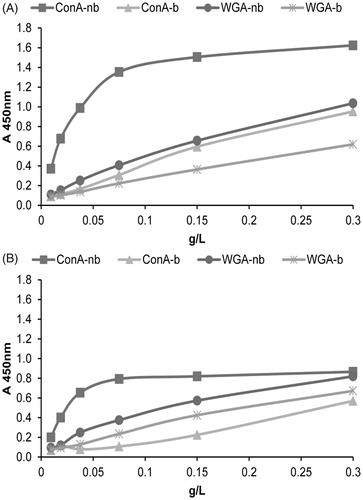
Figure 4. Transmission electron microscopy of seminal prostasomes from oligozoospermic men separated by lectin-affinity chromatography. Micrographs of vesicles (70–120 nm) in a concanavalin A (Con A)-non-bound fraction (A) and vesicles (80–170 nm) in a Con A-bound fraction (B). The insert shows the typical cup-shape appearance enlarged. Micrographs of vesicles (70–130 nm) in a wheat germ agglutinin (WGA)-non-bound fraction (D) and vesicles (50–100 nm) in a WGA-bound fraction (E). Vesicles in the high-pH-eluted fraction from Con A column (C) and low-pH-eluted fraction from WGA column (F) were scarce.
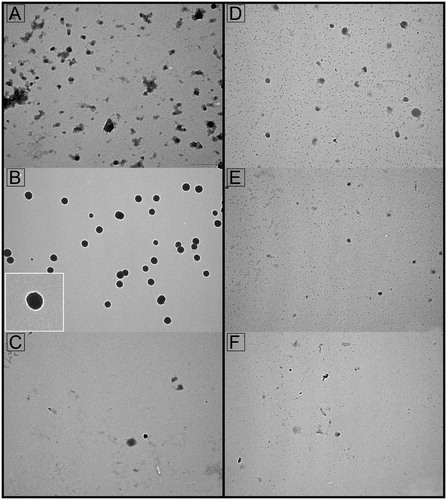
Figure 5. Protein composition of seminal prostasomes from oligozoospermic men separated by lectin-affinity chromatography. Seminal prostasomes separated by lectin-affinity chromatography were resolved on 10% SDS-PAGE under reducing and denaturing conditions and stained with silver. Representative total protein patterns of non-bound (nb) and bound (b) fractions separated on a Con A column and on a WGA column were shown. Samples were loaded as isolated (equal volume/line to reflect yield), and total protein amounts were: line 1 (19 μg), line 2 (10 μg), line 3 (14 μg), line 4 (11 μg), line 5 (50 μg), line 6 (6 μg), line 7 (24 μg), line 8 (13 μg). Characteristic prostasome-associated bands in the region of 90–150 kDa are marked (asterisk). Numbers indicate the position of molecular mass standards (kDa). sPro-O1 and sPro-O2: different isolates of seminal prostasomes from oligozoospermic men.
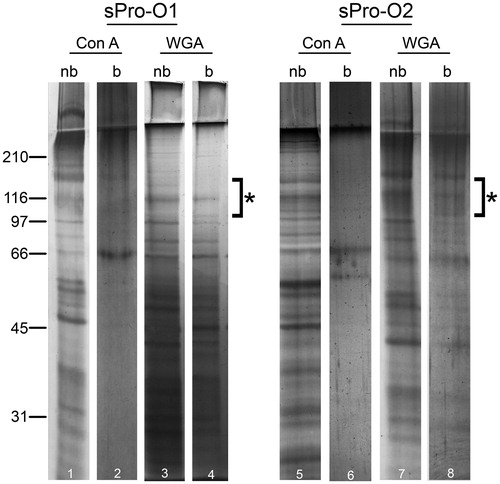
Table 2. Surface-associated markers of seminal prostasomes from oligozoospermic men separated by lectin-affinity chromatography.
Figure 6. Surface glycosylation of seminal prostasomes from oligozoospermic men separated by lectin-affinity chromatography. LAC-separated fractions of seminal prostasomes from oligozoospermic men: Con A-non-bound (Con A-nb), Con A-bound (Con A-b), WGA-non-bound (WGA-nb), and WGA-bound (WGA-b) fractions were immobilized and re-probed with Con A (A) and WGA (B) by solid-phase binding assay.