Figures & data
Figure 1. Overview of the study population. axSpA, axial spondyloarthritis; RA, rheumatoid arthritis; PsA, psoriatic arthritis; ADAb, anti-drug antibodies; DAS28, 28-joint Disease Activity Score; DAPSA28, 28-joint Disease Activity index for PSoriatic Arthritis; NOCB, next observation carried backwards; LOCF, last observation carried forward
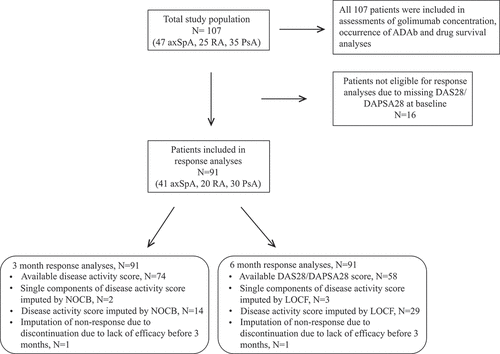
Table 1. Comparison of baseline characteristics in patients with golimumab serum concentration < 1.0 mg/L vs ≥ 1.0 mg/L at 3 month follow-up
Figure 2. Distribution of golimumab serum concentrations in mg/L at 3 months (total inflammatory joint disease population). Median (interquartile range) = 2.2 (1.0–3.5) mg/L
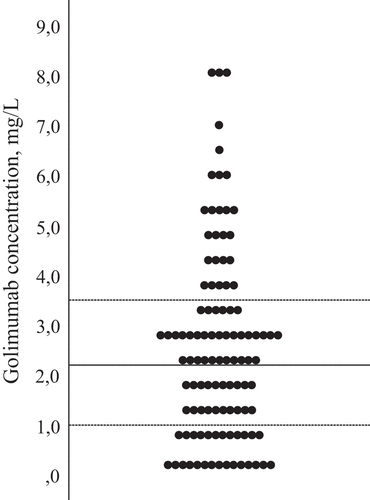
Table 2. Response* (%) at 3 and 6 months, stratified by golimumab concentration at 3 months
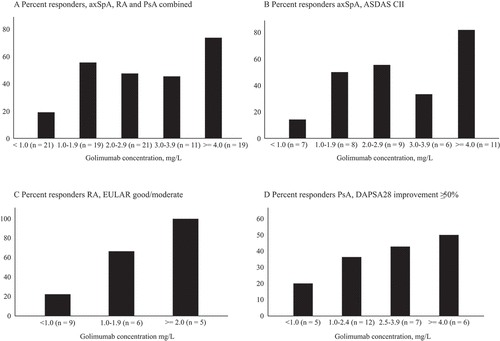
Figure 3. Proportion of responders at 3 months, stratified by golimumab concentration (mg/L). (A) Total inflammatory joint disease population; (B) Ankylosing Spondylitis Disease Activity Score (ASDAS) clinically important improvement (CII) responders in axial spondyloarthritis (AxSpA); (C) European League Against Rheumatism (EULAR) good/moderate response in rheumatoid arthritis (RA); (D) 28-joint Disease Activity Index for PSoriatic Arthritis (DAPSA28) ≥ 50% improvement in psoriatic arthritis (PsA)