Figures & data
Figure 1. Haematological examination of blood from control, lvCIAV-infected, iIBDV-infected and dually lvCIAV/iIBDV-infected groups of SPF chicks. Groups of 18 SPF chicks were infected with lvCIAV at 1 day of age and/or infected with the iIBDV strain 14 days later. Two other groups of 18 1-day-old SPF chicks received PBS and were infected, or not, with iIBDV 14 days later. Haematocrit (PCV) (1a), WBC (1b), percentages of blood heterophils (1c) and lymphocytes (1d) were determined at 18/4, 21/7 and 28/14 days p.i. Values are means of six chicks per group and error bars represent standard deviations. P values were calculated with the ANOVA (post-hoc, Tukey) tests either compared with the control group (*P ≤ 0.05) or with the iIBDV-infected group (†P ≤ 0.05, ††P ≤ 0.01).
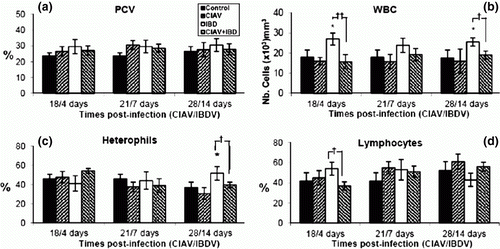
Table 1. Detection of VP3 DNA of lvCIAV and VP2 of iIBDV in thymus, spleen, bursa and caecal tonsils of lvCIAV-vaccinated and/or iIBDV-infected SPF chicks.
Figure 2. Blastic transformation of splenic (2a) and thymic (2b) lymphocytes of control, lvCIAV-infected, iIBDV-infected and dually lvCIAV/iIBDV-infected groups of SPF chicks. Two groups of 18-day-old SPF chicks were infected with lvCIAV and/or with the iIBDV strain 14 days later. Two other groups of 18 1-day-old SPF chicks received PBS and were infected, or not, with iIBDV 14 days later. Splenic and thymic lymphoid cells were stimulated with ConA (100 µg/ml and 1000 µg/ml, respectively) for 72 h. Metabolic activity of these lymphocytes was evaluated by the tetrazolium salt reduction test at 18/4, 21/7 and 28/14 days p.i. Values are means of six chicks per group and error bars represent standard deviations. P values were calculated with the ANOVA (post-hoc, Tukey) tests compared with the control group (*P ≤ 0.05).
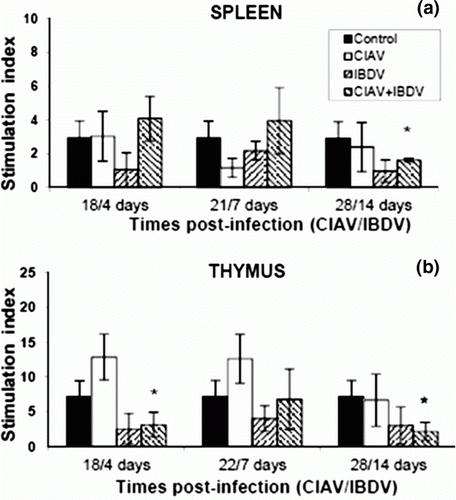
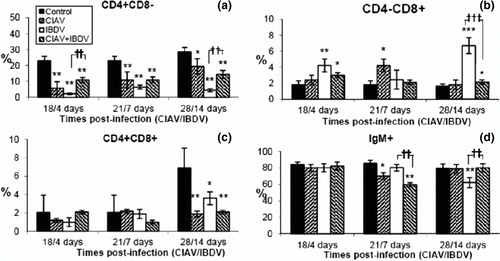
Figure 3. Percentages of lymphocyte subpopulations in the thymus of control, lvCIAV-infected, iIBDV-infected and dually lvCIAV/iIBDV-infected groups of SPF chicks. Groups of 18 SPF chicks were infected with lvCIAV at 1 day of age and/or infected with the iIBDV strain 14 days later. Two other groups of 18 1-day-old SPF chicks received PBS and were infected, or not, with iIBDV 14 days later. Thymic cells were double-labelled with anti-CD4, anti-CD8, anti-CD3, anti-IgM and anti-TCR-γδ antibodies conjugated to FITC or PE and analysed by cytofluorometry. The mean percentages of thymic CD4+CD8− (3a), CD4−CD8+ (3b), CD4+CD8+(3c), and TCR-γδ+ (3d) subpopulations were determined at 18/4, 21/7 and 28/14 days p.i. Values are means of six chicks per group and error bars represent standard deviations. P values were calculated with the ANOVA (post-hoc, Tukey) tests compared with either the control group (*P≤ 0.05, **P≤ 0.01) or the iIBDV-infected group (†P≤ 0.05, ††P≤ 0.01).
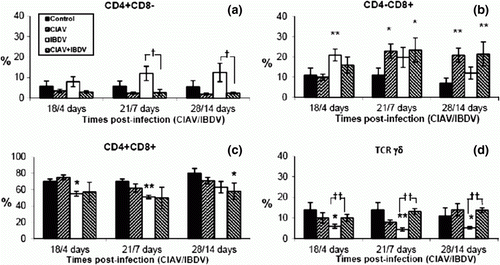
Figure 4. Percentages of lymphocyte subpopulations in the spleen of control, lvCIAV-infected, iIBDV-infected and dually lvCIAV/iIBDV-infected groups of SPF chicks. Groups of 18 SPF chicks were infected with lvCIAV at 1 day of age and/or infected with a iIBDV strain 14 days later. Two other groups of 18 1-day-old SPF chicks received PBS and were infected, or not, with iIBDV 14 days later. Spleen cells were double-labelled with anti-CD4, anti-CD8, anti-CD3, anti-IgM and anti-TCR-γδ antibodies conjugated to FITC or PE and analysed by cytofluorometry. The mean percentage of splenic CD4+CD8− (4a), CD4+CD8− (4b), CD4+CD8+(4c), TCR-γδ+ (4d), IgM+ (4e), and CD3−CD8+ NK cells (4f) subpopulations was determined at 18/4, 21/7 and 28/14 days p.i. Values are means of six chicks per group and error bars represent standard deviations. P values were calculated with the ANOVA (post-hoc, Tukey) tests compared with either the control group (*P≤ 0.05, **P≤ 0.01, ***P≤ 0.001) or the iIBDV-infected group (†P≤ 0.05, ††P≤ 0.01).
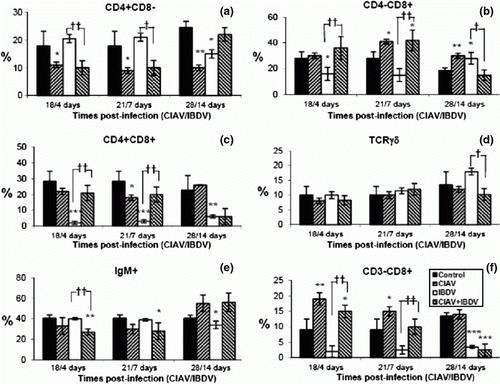
Figure 5. Percentages of lymphocyte subpopulations in the bursa of control, lvCIAV-infected, iIBDV-infected and dually lvCIAV/iIBDV-infected groups of SPF chicks. Groups of 18 SPF chicks were infected with lvCIAV at 1 day of age and/or infected with a iIBDV strain 14 days later. Two other groups of 18 1-day-old SPF chicks received PBS and were infected, or not, with iIBDV 14 days later. Bursal cells were double-labelled with anti-CD4, anti-CD8, anti-CD3 and anti-IgM antibodies conjugated to FITC or PE and analysed by cytofluorometry. The percentages of bursal CD4+CD8− (5a), CD4−CD8+ (5b), CD4+CD8+(5c), and IgM+ (5d) subpopulations were determined at 18/4, 21/7 and 28/14 days p.i. Values are means of six chicks per group and error bars represent standard deviations. P values were calculated with the ANOVA (post-hoc, Tukey) tests compared with either the control group (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001) or the iIBDV-infected group (†P≤ 0.05, ††P≤ 0.01, †††P≤ 0.001).