Figures & data
Table 1. Background information of M. synoviae field isolates (n = 194), MS-H-live vaccine (n = 3) and other avian mycoplasma strains (n = 43) used for the development and validation of the differentiating M. synoviae qPCR.
Table 2. Results of the field validation of the differentiating M. synoviae qPCR and of MLST of trachea mucus samples from broiler breeders (pooled swabs) and layers (individual swabs).
Figure 1. Sampling scheme, housing and results of the M. synoviae RPA test and the differentiating M. synoviae qPCR of a non-M. synoviae-vaccinated broiler breeder flock (farm K) and the rearing flock of origin (farm H). At the rearing farm, birds kept in two adjoining houses (5 and 6) had been vaccinated with MS-H at 10 weeks of age. At approximately 18 weeks of age, birds from rearing houses 1, 2, 3 and 4 were transferred to farm K, while those from houses 5 and 6 were transferred to another production farm. Shortly before transfer, the non-vaccinated birds showed negative RPA test results, while the vaccinates had seroconverted. All broiler breeders of farm K sampled at 23 and 25 weeks had seroconverted also, after which trachea swabs were collected for analysis with the differentiating M. synoviae qPCR. The latter showed the presence of MS-H, field M. synoviae or mixed infections in a substantial number of trachea mucus samples. These results suggest that spread of MS-H had occurred at the rearing farm, while birds of farm K also had been infected with field M. synoviae. The number of birds per house at the rearing farm, the number of blood samples for serology and the number of trachea swabs for qPCR analysis are indicated.
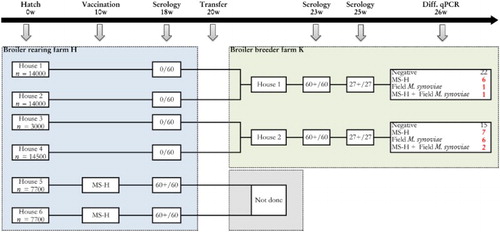
Figure 2. Sampling scheme, housing and results of the M. synoviae RPA test and the differentiating M. synoviae qPCR of a non-M. synoviae-vaccinated broiler breeder flock (farm B) and the rearing flock of origin (farm G). At the rearing farm, birds kept in two adjoining houses (3 and 4) had been vaccinated with MS-H at 10 weeks of age. At approximately 18 weeks of age, birds from rearing houses 1 and 2 were transferred to farm B, while those from houses 3 and 4 were transferred to another production farm. Shortly before transfer, the non-vaccinated birds showed negative RPA test results, while the vaccinates had seroconverted. All broiler breeders of farm B sampled at 24 and 31 weeks had seroconverted also, after which trachea swabs were collected for analysis with the differentiating M. synoviae qPCR. The latter showed the presence of MS-H in a substantial number of trachea mucus samples. These results suggest that spread of MS-H had occurred at the rearing farm. The number of birds per house at the rearing farm, the number of blood samples for serology and the number of trachea swabs for qPCR analysis are indicated.
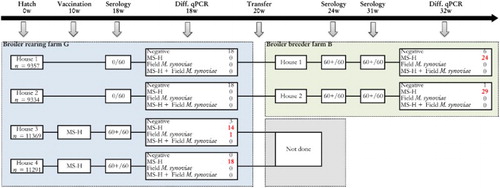
Figure 3. Sampling scheme, housing and results of the M. synoviae RPA test and the differentiating M. synoviae qPCR of a non-M. synoviae-vaccinated layer flock (farm O), a vaccinated layer flock (farm D) and the rearing flock of origin (farm B). At farm B, rearing layers were kept in one house with two sections (1a and 1b) separated only by a wire mesh. The birds in section 1b had been vaccinated with MS-H at 11 weeks of age. At approximately 18 weeks of age, birds from rearing house 1a were transferred to farm O, while those from house 1b were transferred to a layer farm D. The latter farm was located at 25 meters distance from another layer farm infected with field M. synoviae. Shortly before transfer, both the non-vaccinated as well as the vaccinates were positive for MS-H in the differentiating M. synoviae qPCR, suggesting that spread of MS-H had occurred at the rearing farm. All layers sampled on layer farm O (21 weeks of age) and D (21, 25 and 29 weeks of age) and the neighbouring farm (61, 68 and 72 weeks of age) had seroconverted, after which trachea swabs were collected for analysis with the differentiating M. synoviae qPCR. The latter showed the presence of MS-H in a substantial number of trachea mucus samples from farm O and D. At week 72 of age, field M. synoviae was also detected in farm D indicating that birds had been infected with M. synoviae field strain. In trachea mucus samples collected from birds at the neighbouring farm only field M. synoviae was detected using the differentiating M. synoviae qPCR. The number of birds per house at the rearing farm, the number of blood samples for serology and the number of trachea swabs for qPCR analysis are indicated.
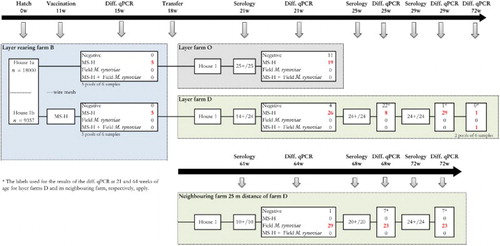
Figure 4. Phylogenetic tree of 35 M. synoviae field isolates and the MS-H vaccine strain including its re-isolates constructed from the concatenated sequences of upgA, uvrA, lepA, nanA and ruvB genes, which are used for the M. synoviae MLST. Samples in which only MS-H (♦) or field M. synoviae (•) or both, MS-H and field M. synoviae (□) were detected by the differentiating M. synoviae qPCR are indicated with the corresponding symbols. Sample identification (indicated by MS and a figure or additional text) corresponds to the sample identification used in . ST sequence type.
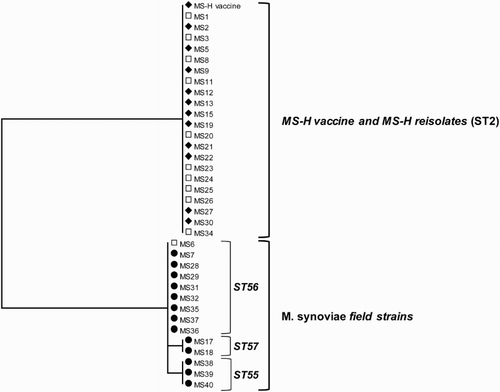
Figure 5. Quantitative results of the differentiating M. synoviae qPCR performed on trachea swabs of birds from houses 1 and 2 of the broiler breeder production farm K. Field M. synoviae (◊), MS-H (♦) and mean quantitative results for field M. synoviae (□) and MS-H (▪) are indicated.
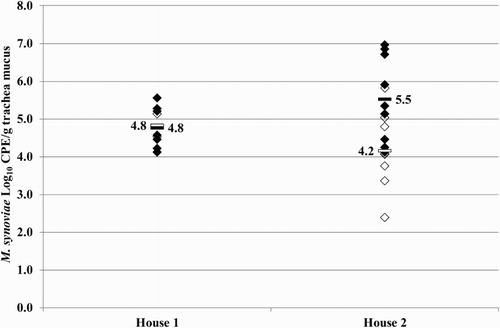
Figure 6. Quantitative results of the differentiating M. synoviae qPCR performed on trachea swabs of birds from broiler breeder farm B. MS-H (♦) and mean quantitative results (▪) are indicated.
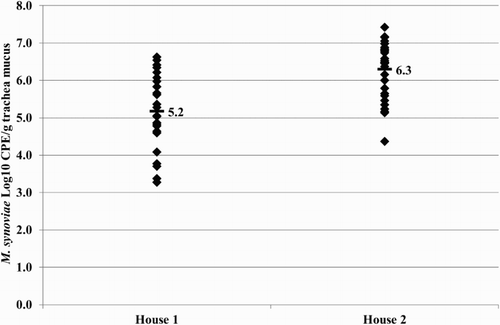
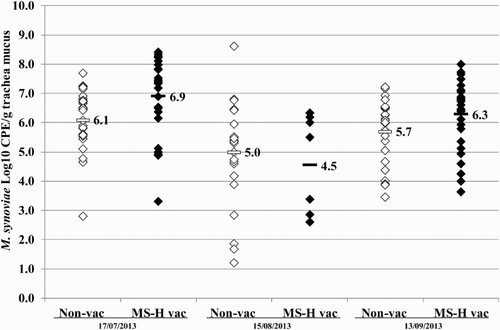
Figure 7. Quantitative results of the differentiating M. synoviae qPCR performed on trachea swabs of birds from layer farm D (MS-H vaccinated (MS-H vac)) and the neighbouring layer farm (non-vaccinated (non-vac)) at three different time points. Field M. synoviae (◊), MS-H (♦) and mean quantitative results for field M. synoviae (□) and MS-H (▪) are indicated.