Figures & data
Figure 1. Schematic view of the HVT-FC126 cosmid library containing overlapping DNA fragments of the virus and the insertion vector used to create HVT-NDV-ILT. The ILT gD plus gI fragment and NDV-F expression cassette were cloned into the US2 region of HVT US insertion vector.
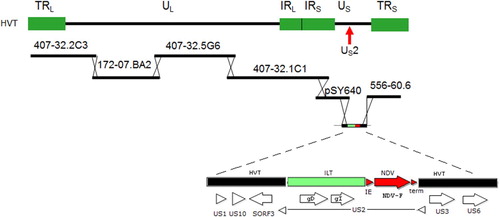
Table 1. List of primers used for PCR.
Figure 2. Photographs of HVT-NDV-ILT plaques stained with antibodies specific to the three inserted gene products. (A) HVT-NDV-ILT plaque stained with anti-ILT gD monoclonal antibody, MAB 6, followed by a FITC labelled goat anti-mouse IgG. (B) HVT-NDV-ILT plaque stained with a rabbit anti-ILT gI polyclonal antibody followed by a FITC labelled goat anti-rabbit IgG. (C) HVT-NDV-ILT plaque stained with an anti-NDV-F monoclonal antibody, 57NDV, followed by a FITC labelled goat anti-mouse IgG.
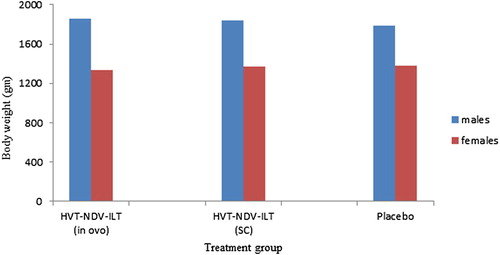
Figure 3. The safety of the double recombinant vaccine HVT-NDV-ILT, in terms of its potential to cause changes in body weights in chickens, was evaluated in an overdose study using SPF birds as described under materials and methods. At the time of necropsy (120 days of age), the mean body weight for each of the treatment groups was computed with the GLM procedure in SAS® 9.1.3. There was no statistically significant difference in the mean bird body weight between the vaccinated groups and the Marek’s vaccine diluent inoculated group. Left hand bars for each treatment group represent males, right females.