Figures & data
FIGURE 1. Release profiles of diltiazem hydrochloride from extruded pellets based on various polymers (polymer/drug ratio 1:1, size 2 × 2 mm). (▪) EC; (Δ) CAB; (⋄) Eudragit®; (•) EVAC.
FIGURE 2. The percentage phenylpropanolamine (PPA) released at 6 h from tablets containing 15% PPA, 30% Precirol, and 55% filler excipients prepared by hot-melt extrusion (HME) and high-shear melt granulation (MG). (□) Tablets compressed from granules within 20–40 mesh only; (▪) Tablets compressed from granules within 60–100 mesh only; (![]()
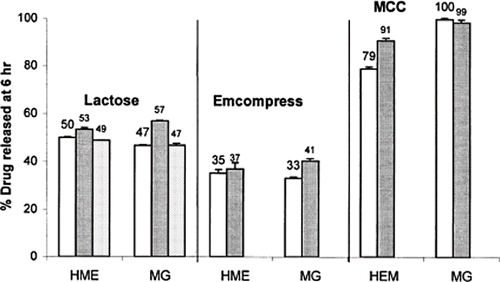
FIGURE 3. Stability of guaifenesin release rate from melt-extruded pellets (A) With out ethyl cellulose (B) With ethyl cellulose upon storage at 40C/75%RH in sealed HDPE containers with silica desiccants. Key: ▴ Initial; Δ 1 Month; ▪ 3 Months.
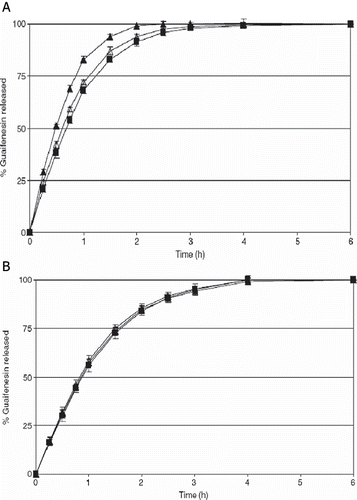
TABLE 1. Mean Pharmacokinetic Parameters (± SD) After Oral Administration of 300 mg Ibuprofen to Healthy Volunteers (n = 9): Ibu-slow® 600 (1/2 tablet), Mini-matrix F-1 Containing HPMC and Mini-matrix F-2 Containing Xanthan Gum
TABLE 2. Pharmacokinetic Parameters Calculated Using Noncompartmental Analysis in Win-nonlin of Rats Dosed with Micronized Particle Extrudate (MPE) Compositions and Crystalline ITZ
FIGURE 4. Relationship of polymer molecular weight in the thermal stability of PEO when stored at 60°C, 75% relative humidity, (♦) 1,000,000, (▪) 600,000 and (▴) 200,000.
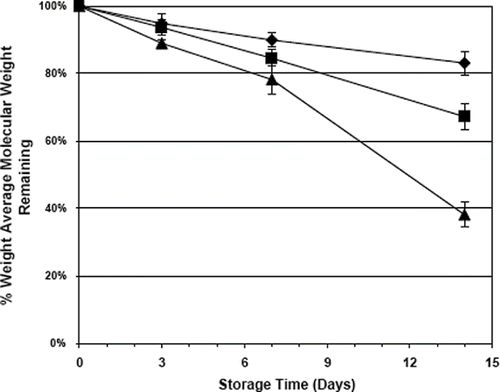
TABLE 3. Extrusion Stability of PEO 1M and the Influence of PEO 100K on PEO Weight Average Molecular Weight as Measured by Gel Permeation Chromatography, ± SD, n = 3
FIGURE 5. Influence of TEC concentration and pre-plasticization on the drug release rate of hot-melt extrudate tablets containing 25% w/w 5-ASA. (▴) Formulation pre-plasticized with 12% w/w TEC; (♦) Formulation, not pre-plasticized with TEC; (▪) Formulation, pre-plasticized with 23% TEC.
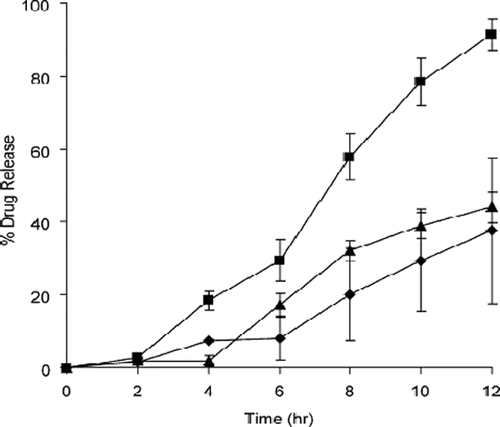
FIGURE 6. Morphologies of surface and cross-sectional floating HME tablets prepared from formulation containing 10% sodium bicarbonate: (A) Surface 50x, (B) Surface 200x, (C) Cross-sectional 50x, (D) Cross-sectional 200x.
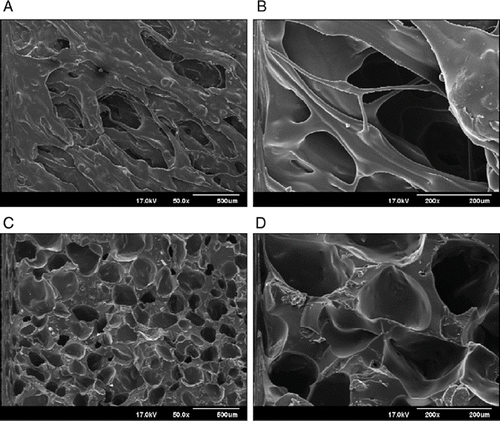
FIGURE 7. CPM release profiles from (A) DC tablets and (B) HME tablets in 900 mL of either (♦) 0.1N HCl, (⋄) pH 4.0 acetate buffer, (▴) pH 4.0 citrate buffer, (Δ) pH 4.0 phosphate buffer, (▪) pH 6.8 phosphate buffer, or (□) pH 7.4 phosphate buffer at 37 ± 0.5°C (USP 27 apparatus 2, 100 rpm). Each point represents the mean ± S.D., n = 3.
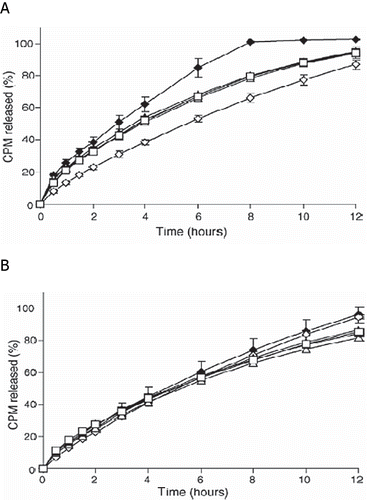
FIGURE 8. Glass transition temperatures of HPC/PEO (50:50) hot-melt extruded films containing vitamin E TPGS and three conventional plasticizers (n = 4).
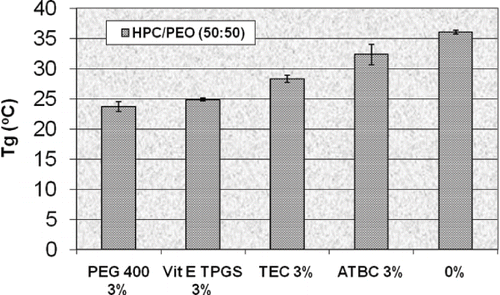
FIGURE 9. (a) Tensile strength and (b) Percent elongation of HPC/PEO 50:50 rRatio hot-melt extruded films containing vitamin E TPGS and three conventional plasticizers (n = 6).
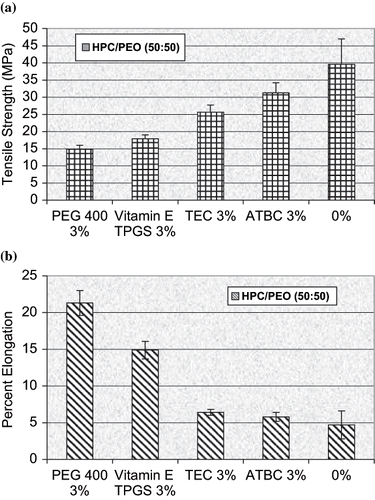
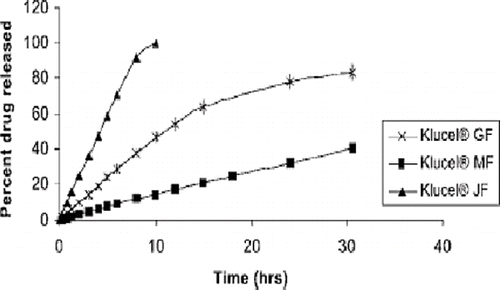
FIGURE 10. Release profiles of clotrimazole from hot-melt extruded Klucel® films as a function of polymer molecular weight.