Figures & data
Figure 1. IND date availability per year. Notes: IND dates were identified and collected from two primary public sources: the Drugs@FDA database and the Federal Register. The availability of dates varied over time, both by total availability of IND dates year-to-year and availability by source.
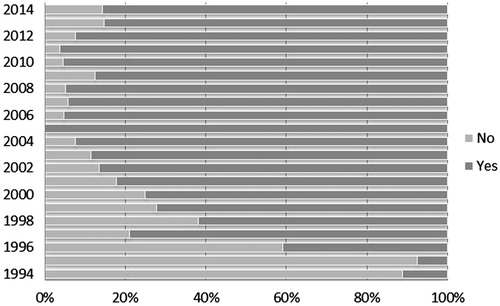
Figure 2. IND date availability per data source. Notes: IND dates were identified and collected from two primary public sources: the Drugs@FDA database and the Federal Register. The availability of dates varied over time, both by total availability of IND dates year-to-year and availability by source.
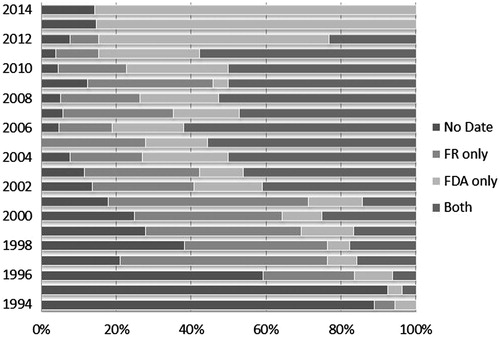
Table 1. Number of NMEs by date entry and data source.
Table 2. Gaps between multiple dates reported for the same NME.
