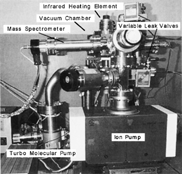
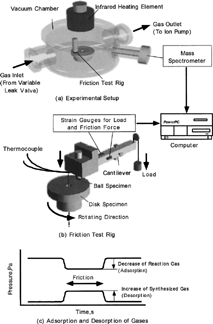
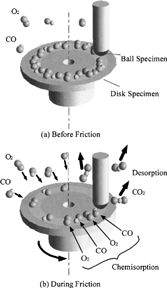
Figures & data
FIG. 6 Relationship between coefficient of friction and chemisorption or desorption rate of gases, O2, CO; 1.0 × 10−4 Pa.
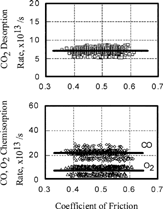
FIG. 7 Relationship between coefficient of friction and chemisorption or desorption rate of gases, O2, CO; 5.0 × 10−4 Pa.
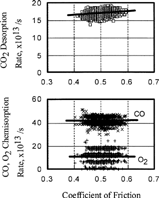
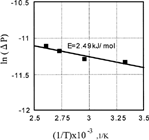
FIG. 8 Effect of CO and O2 pressure on the ratio of O2 chemisorption rate over coefficient of friction.
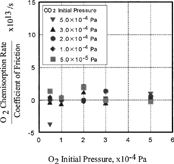
FIG. 9 Effect of CO and O2 pressure on the ratio of CO chemisorption rate over coefficient of friction.
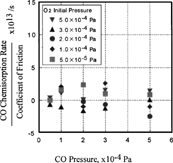
FIG. 10 Effect of CO and O2 pressure on the ratio of CO2 desorption rate over coefficient of friction.
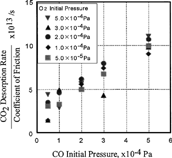
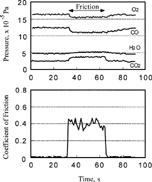
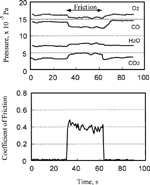
FIG. 11 Pressure change of carbon monoxide, oxygen, and carbon dioxide at the start and the end of sliding.
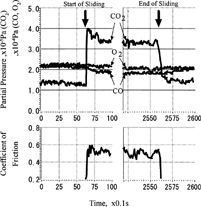
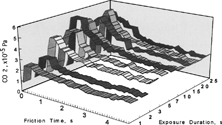
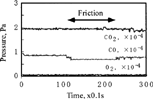
FIG. 12 Effect of exposure duration before friction on CO2 pressure (CO and O2; 5.0 × 10−5 Pa), sliding velocity = 93 mm/s.

FIG. 13 Effect of exposure duration before friction on CO2 pressure (CO and O2; 1.0 × 104 Pa), sliding velocity = 93 mm/s.