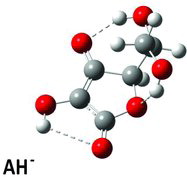
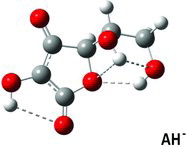
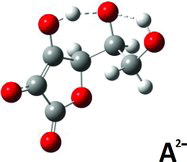
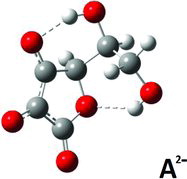
Figures & data
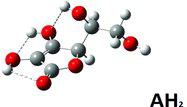
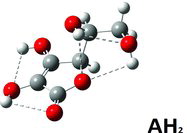
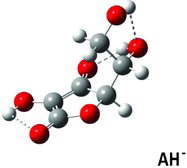
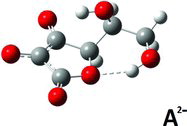
Table 1 Selected structure data in solved crystal structures compared to DFT calculateda results for AH2, AH−, and A2−
Table 2 Intramolecular hydrogen bond distances for AH2, AH−, and A2− molecules in different minimizeda conformations
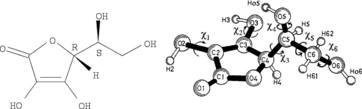
Table 3 Calculated dihedral (torsion) angles χ1, χ3–χ6 for the ascorbate ion minimum energy conformer (Figure 5) compared with similar angles in selected crystal structuresa
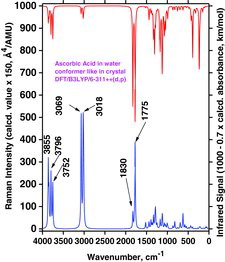
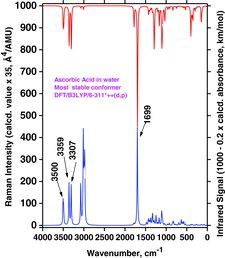
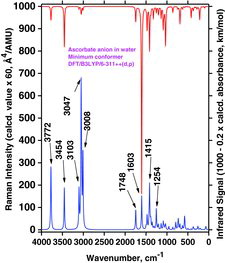
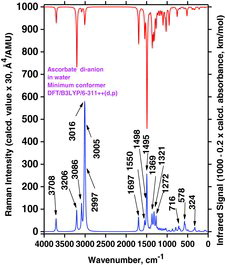
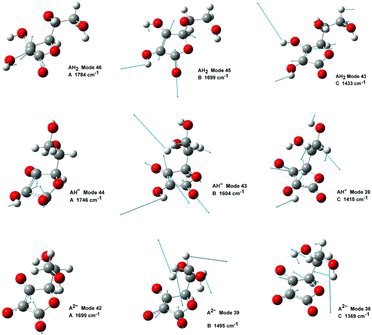
Table 4 Selected wavenumber dataa for IR absorption and Raman scattering strong bands of AH2, AH−, and A2− compounds dissolved in water. Data were calculated by the Gaussian 03/DFT/B3LYP/6-311++G(d,p)/PCM
Table 5 Selected model results for ions AH− and A2− in water (PCM)a,b
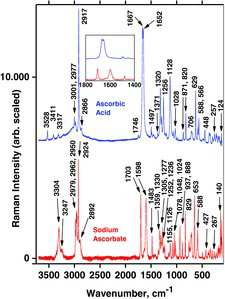
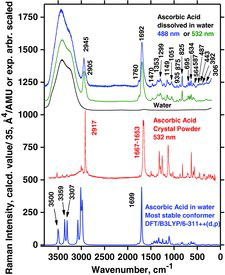
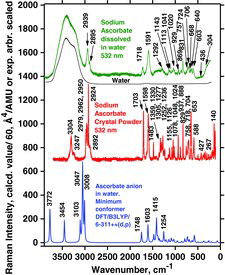
Table 6 Measured Raman spectral bands (in cm−1) and assignments for ascorbic acid and sodium ascorbate solids, compared to literature values (39, 44–46, 48). Some data can also be found in Jehlička et al. (Citation47)
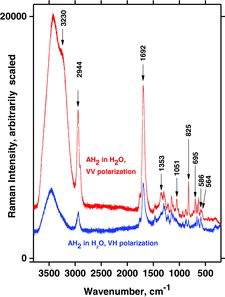
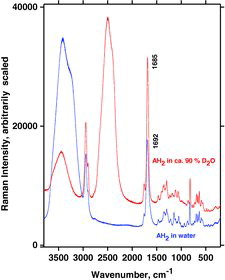
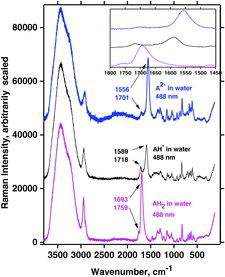
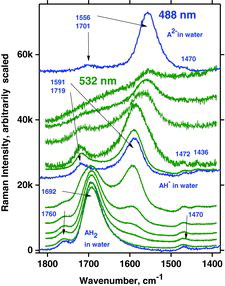
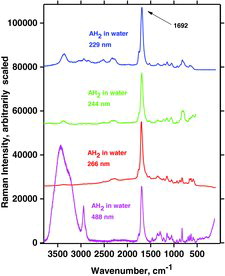
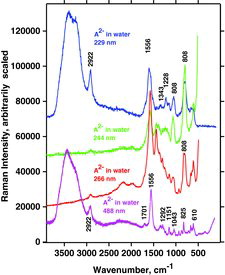
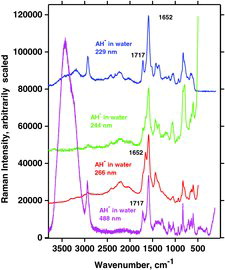
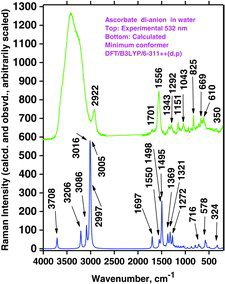
Table 7 Measured Raman spectral bands (in cm−1) and assignments for aqueous solutions of ascorbic acid and sodium ascorbates, compared to literature values (39, 44, 46, 48). Some data can be found in Jehlička et al. (Citation47). Water bands are not included. For abbreviations, see Table 6
Table 8 Wavenumber values (cm−1) and calculated Raman intensity (int, in units of Å2/amu) of vibration bands A, B, and C for AH2, AH−, and A2− in water. Observed wavenumber values (Obsd.) from Table 7. For intensity abbreviations see Table 6
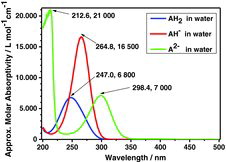
Table 9 Ultraviolet absorption data of ascorbic acid species; see Figure 27