Figures & data
Figure 1. A, Location of the study area in the Bas-Saint-Laurent region, Québec, Canada, with the geological and hydrogeological context of the study area. B, Schematic drawing of the principal hydrostratigraphic units present along cross section 1–2, from the coastal plain to the highlands.
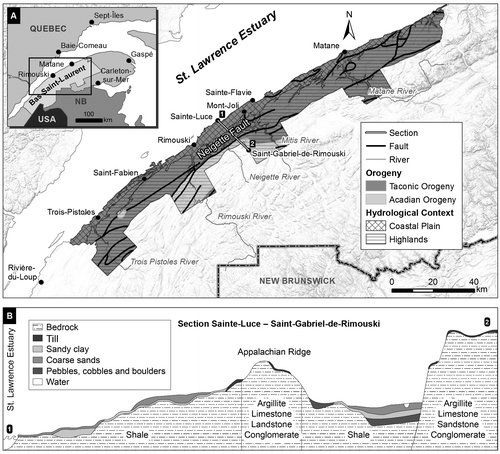
Figure 2. Piper diagram showing water chemistry based on major ions in the granular and bedrock aquifers. The four geochemical groups identified by hierarchical cluster analysis (HCA) are reported. Characteristics of seawater (X) are presented in the diagram.
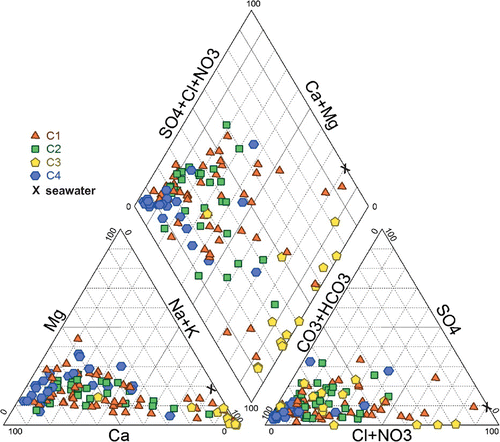
Table 1. Contribution of the different geochemical facies as revealed by the Piper diagram.
Table 2. Mean geochemical and physical parameter values characterizing the four geochemical groups identified by hierarchical cluster analysis (HCA).
Figure 3. Locations of the four geochemical water groups based on 11 parameters as revealed by a hierarchical cluster analysis (HCA). The two main hydrological contexts (the coastal plain and the highlands) and the degree of confinement of Bas-Saint-Laurent (BSL) aquifers are also reported.
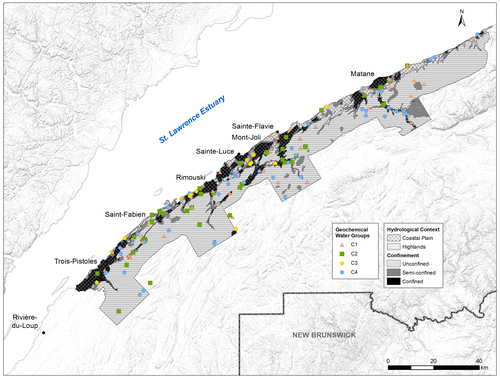
Figure 4. Distribution of δ18O and δ2H (V-SMOW in ‰) in groundwater facies. The dotted line represents the line linking the stable isotopes of solid and liquid precipitation, and the black line is the linear regression of groundwater samples. The mean values of δ18O and δ2H are also reported for the regional rain and snow precipitation and seawater (data from Buffin-Bélanger et al. Citation2015 and Chaillou et al. Citation in press).
Figure 5. Relationships between different ions and Cl content (in mg/L) in Bas-Saint-Laurent (BSL) groundwater. The grey lines represent the theoretical mixing line between fresh groundwater and seawater for Na (A), Mg (B), Ca (C), and NO3 (D). The high-mineralized sample is reported in parentheses.
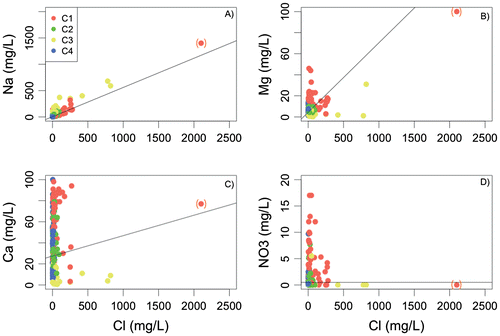
Figure 6. A, Sodium concentrations; B, bromide to chloride; and C, sulphate to chloride mass ratios reported as a function of chloride concentrations. Marine mass ratios are also reported. The high-mineralized sample is not reported.
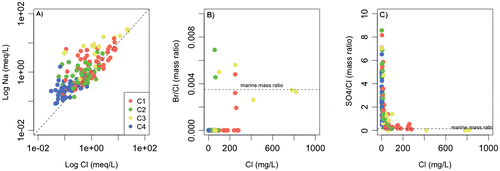
Figure 7. Processes related to water–rock interaction reported for the four water groups: Ca (in meq/L) (A) and pH (B) in relation to Na (in meq/L). The high-mineralized sample is not reported.
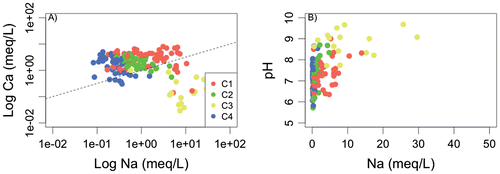
Figure 8. A, Calcium content (in meq/L) in relation to bicarbonate (HCO3 in meq/L). B, Sum of major cations (Ca + Mg in meq/L) in relation to bicarbonate (HCO3 in meq/L). C, Sum of major ions (Ca + Mg − (HCO3+SO4) in meq/L) in relation to Na-Cl (in meq/L). The high-mineralized sample is not reported.
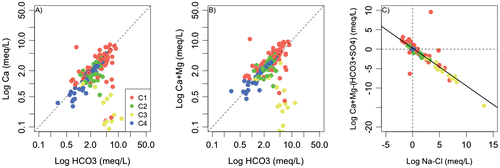
Figure 9. Saturation indexes (SI) of carbonate minerals. A, SI of calcite in relation to pH; and B, relationship between calcite and dolomite SI. The high-mineralized sample is not reported.
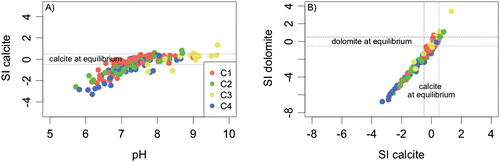
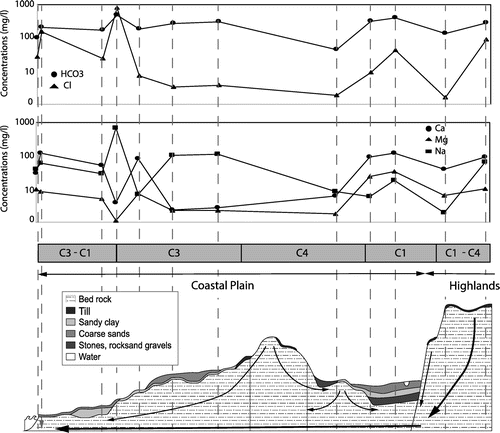
Figure 10. Conceptual model of the hydrogeochemical evolution of groundwater in the Bas-Saint-Laurent (BSL) bedrock aquifers along cross section 1–2 (see location in Figure A and geological details in Figure B). Concentrations of major anions (A) and cations (B) along the section (concentrations are reported in mg/L). C, Geology, hydrological settings, and expected groundwater flow paths from the recharge area to the coastal discharge zone. The water groups are reported as a function of the ion concentrations.