Figures & data
Table 1. Primer sequences, temperature (Tm; °C), and number of cycles used for polymerase chain reactions
Fig 1. (a) RT-PCR analysis of VdNEP expression in V. dahliae cultures from 3 to 40 days; Vd1396-9 and Vd1398-21 are highly aggressive on sunflower whereas Vs06-07 and Vs06-14 are weakly aggressive isolates. VdNEP was amplified by 30 cycles. (b) RT-PCR analysis of the expression of VdNEP in sunflower roots one week after inoculation with V. dahliae. IS6111 and IS8048 are moderately resistant and susceptible lines, respectively. Lane M represents the DNA ladder. Lane Ctrl(+) is a positive control of VdNEP gene from Vd1396-9 whereas lane Ctrl(-) is a negative control amplified from wounded sunflower roots. Transcripts of Tubulin from V. dahliae were used as an internal control.
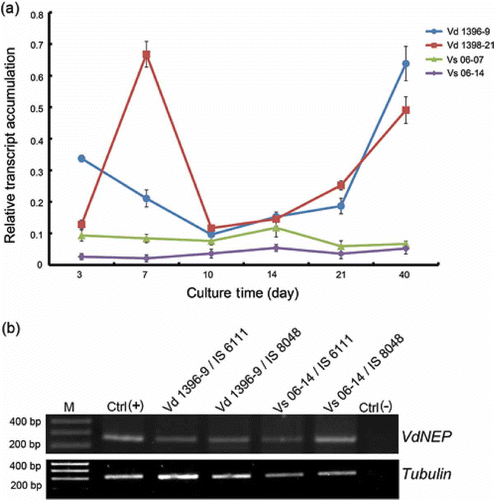
Fig 2. Double digestion of pET-32a (+) with VdNEP insert by restriction enzymes EcoR I and NOT I (a); SDS-PAGE of VdNEP protein expressed in pET-32a (+)/BL21 E. coli strain (b); Lane M, protein size standard in kDa; lane S, total protein extracts from supernatant of BL21 after sonification; Lane F, protein extracts flowing through from His Bond Resin; Lane W, protein extracts washed by 20 mM imidazole; and Lane E, purified His-VdNEP protein eluted by 250 mM imidazole; Protein gel blot analysis showing the secretion of VdNEP in Vd1396-9 cultured for 14 days (c); Lane M, size standards in kDa; Lane 1, purified His-VdNEP used as the positive control; Lanes 2 and 3, native VdNEP proteins extracted from liquid culture and mycelium of Vd1396-9, respectively. In the western blot, His-VdNEP protein (first lane) is a positive control to make sure the polyclonal antibody worked well. Lane 2 and 3 represent the native VdNEP protein secreted from V. dahliae isolate. The expressed His-VdNEP protein in vitro is bigger than native protein in vivo, as indicated by the small difference in their sizes.
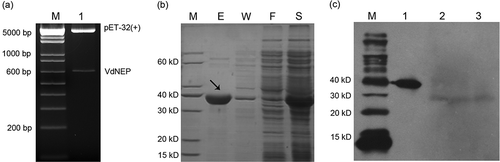
Fig 3. Vascular discoloration (a) and disease severity caused by V. dahliae and His-VdNEP five weeks after inoculation (w.a.i.) in moderately resistant IS6111 (b) and susceptible IS8048 (c) sunflower hybrids, respectively.
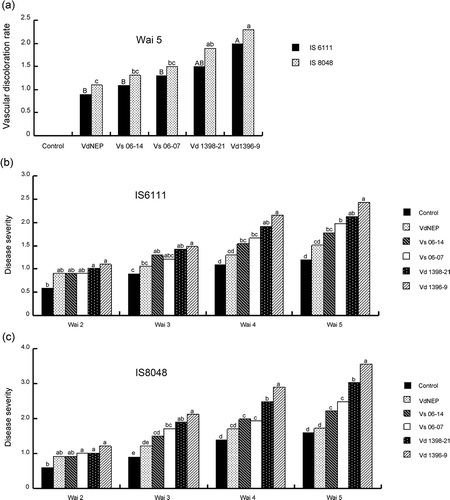
Fig 4. (a) Chlorosis and necrosis in leaves of sunflower hybrids IS6111 and IS8048, induced by His-VdNEP (20 μg mL−1) and V. dahliae isolates three weeks after inoculation (w.a.i.) by root dipping; and (b) percentage of cell death in sunflower leaves treated with V. dahliae and His-VdNEP (20 μg mL−1). The error bars represent the standard error.
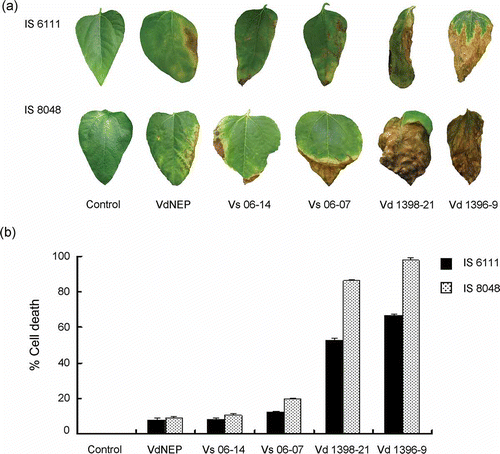
Fig 5. Hypersensitive response in N. benthamiana leaves (a) and sunflower IS 6111 cotyledons (b) induced by His-VdNEP 24 h after infiltration; a1 and a4 are negative controls treated with DDW or His tag protein (20 μg mL−1); a2, a3, a5 and a6 are treatments with 10, 20, 200 and 2000 μg mL−1 His-VdNEP, respectively; (c) DNA laddering of 180–200 bp DNA fragments in sunflower IS 6111 roots inoculated with His-VdNEP; Lanes: M, 1 Kb Plus DNA Ladder; 1 and 2, negative control treated with DDW or His tag protein (20 μg mL−1), respectively; Lane 3, 4, 5 and 6, treatments with His-VdNEP at 0.05, 0.5, 5 or 20 μg mL−1, respectively; (d) Accumulation of fluorescent compounds and (e, f) oxidative burst in sunflower IS6111 leaves inoculated with His-VdNEP. Pictures in (d, e) were taken under 10×/20× magnification. The production of reactive oxygen species activated by His-VdNEP (20 μg mL−1) was examined after 3,3-diaminobenzidine staining of tissues 6 h after infiltration; CK are sunflower leaves treated with His tag protein (20 μg mL−1).
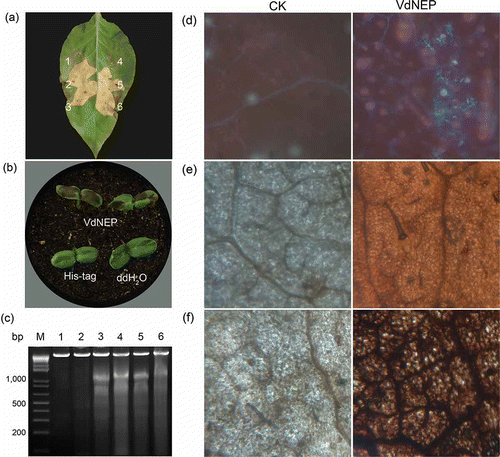
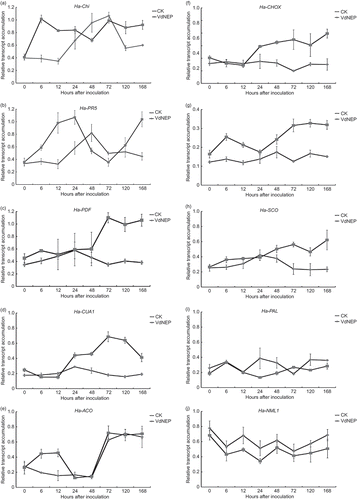
Fig 6. Expression of Ha-Chi (a), Ha-PR-5 (b), Ha-PDF (c), Ha-CUA1 (d), Ha-ACO (e), Ha-CHOX (f), Ha-GST (g), Ha-SCO (h), Ha-PAL (i) and Ha-NML1 (j) in sunflower plants inoculated with His-VdNEP (20 μg mL−1). CK represent controls inoculated with water. Leaves were collected from 0 to 168 h after inoculation (h.a.i.). Three biological replicas were included and the error bars represent the standard error.