Figures & data
Table 1. Summary of REML estimates for the random effects (also expressed as a percentage of total variance) of the AUDPC and balanced repeated measures analysis for Experiment 1
Table 2. Summary of REML estimates for the random effects (also expressed as a percentage of total variance) for the balanced repeated measures analysis used to assess disease reaction to Diaporthe toxica when applied as droplets to leaflets of Lupinus albus in a detached leaf assay (Experiment 2)
Fig. 1. Effect of spore concentration of phomopsis leaf blight symptoms (caused by Diaporthe toxica) on detached leaves of Lupinus albus expressed as increase from the control. Comparison between two analyses: either balance repeated measures or calculated area under disease progress curve ().
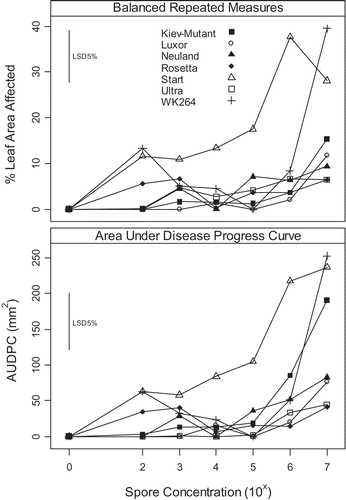
Fig. 2. (A) Effect of spore concentration on expression of phomopsis leaf blight in Lupinus albus using a detached leaf assay. (B) ‘Kiev-Mutant’ sprayed with spore concentration 1 × 107 Diaporthe toxica spores ml−1 7 days after inoculation showing actively sporulating pycnidia. (C) Pycnidia formation on a dried leaflet in a glasshouse screening experiment, data reported elsewhere (Cowley et al., Citation2010). Bars approximately equal 10 mm.
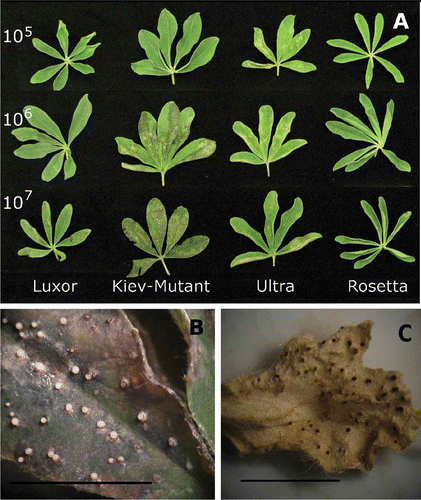
Fig. 3. Effect of leaf position and volume of spore suspension (at 1 × 107 Diaporthe toxica spores ml−1) applied to detached leaves of Lupinus albus in Experiment 2. Leaf 1 was the first true leaf and the next leaves (up to five) were taken in successive order up the stem from the cotyledons.
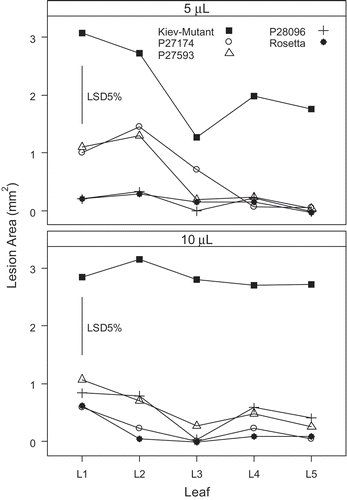
Fig. 4. Development of foliar lesions after inoculating Lupinus albus seedling leaves with 5 μL of a 1 × 107 spore suspension of Diaporthe toxica. Bars with the same letters are not significantly different (P < 0.05).
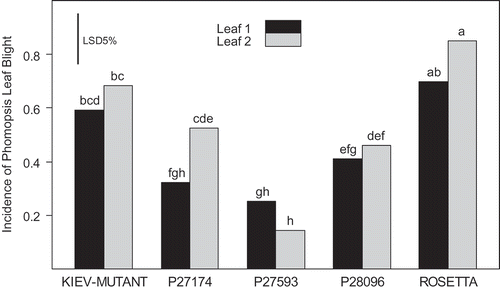
Fig. 5. Predicted means using a detached leaf assay for a diverse selection of Lupinus albus genotypes screened for resistance to phomopsis leaf blight caused by Diaporthe toxica in a screening experiment. Genotypes with white bars are the five parents of existing mapping populations used in Experiments 2 and 3. The grey bars highlight genotypes considered resistant.
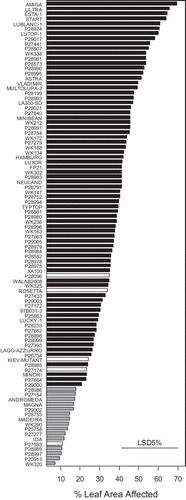
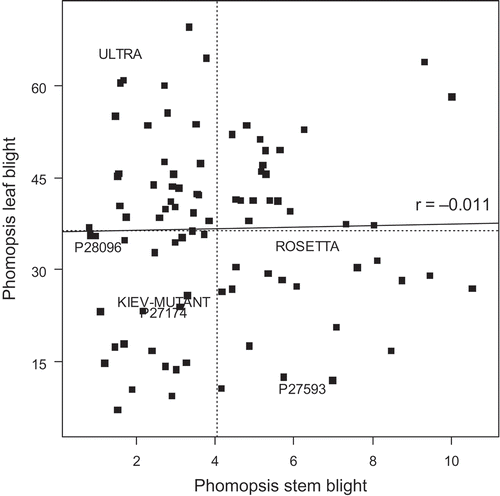
Fig. 6. Correlation between phomopsis leaf blight of Lupinus albus using a detached leaf assay (% leaf area affected) and phomopsis stem blight (square-root of lesion length). The same plants were used in both assessments. The horizontal and vertical dotted lines indicate the mean values for phomopsis leaf blight and phomopsis stem blight respectively. The solid line is the correlation line between the two measures. Genotype names (where used) are centred on the data position.