Figures & data
Table 1. ELISA, RT-PCR and bioassay on potato ‘Kennebec’ leaf samples collected in a field plot in July 2011
Fig. 2. Reverse transcription–polymerase chain reaction (RT–PCR) detection and genotyping of Potato virus Y (PVY) in field samples. A. P1-gene based duplex RT–PCR for determination of PVY° and PVYN/N:O/NTN. B. Recombinant joint (RJ)-based multiplex RT–PCR for detection of recombinant PVYN:O and PVYNTN. C. Multiplex RT-PCR for detection of PVY°, PVYN:O, PVYNTN and PVYN strains. D. Schematic diagram of PVY genome structure and locations of P1 gene, and RJ1 to RJ3. The RT–PCR assays A and B were carried out as described in Nie & Singh (Citation2002, Citation2003), and assay C as described in Lorenzen et al. (Citation2006). The sizes of the amplicons produced by different PVY strains in assay C are: 689 + 267 bp for PVY°; 452 + 181 bp for recombinant PVYNTN (Lorenzen et al., Citation2006). Lane M, DNA Ladder (from top to bottom: 2000, 1200, 800, 400 and 200 bp); lanes 1 to 14, leaf samples collected from potato ‘Kennebec’ plants showing varied degrees of symptoms in the field.
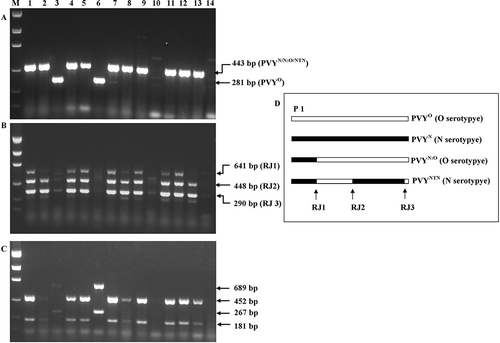
Fig. 1. Symptoms in potato ‘Kennebec’ in the field and tobacco- and potato ‘Yukon Gold’-based bioassay for unveiling symptom-causal agents in ‘Kennebec’ plants. A. Symptoms in ‘Kennebec’ in the field. B and C. Tobacco symptoms induced by the corresponding field samples (inoculum) shown in A at 7 (B) and 21 (C) days post inoculation. D. ‘Yukon Gold’ tuber symptoms induced by field PVY isolates obtained from the corresponding samples shown in A. One symptomless (# 14) and 13 symptomatic plants (# 1–13) were photographed and sampled in July 2011. The samples were used as inocula to sap-inoculate tobacco seedlings (B and C) or virus-free (VF) plantlets of potato ‘Jemseg’ (photos not shown), which in turn were used as inocula to inoculate VF ‘Yukon Gold’ plantlets. Tubers harvested from inoculated ‘Yukon Gold’ plants are shown in D.
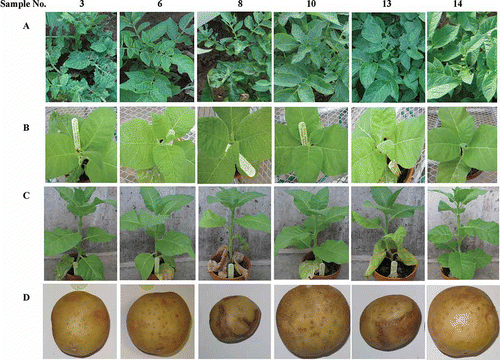
Table 2. Response of potato ‘Kennebec’, tobacco ‘Samsun’ and Physalis floridana to PVX and/or PVY infections in the greenhouse
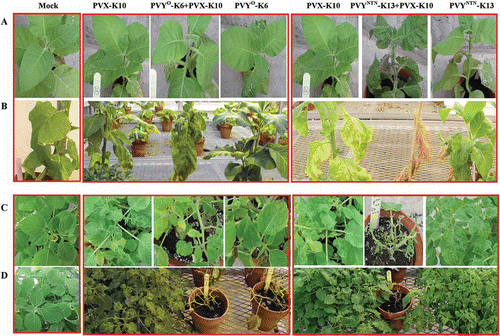
Fig. 3. Systemic symptoms caused by single or double infections with Potato virus X (PVX) and Potato virus Y common strain (PVY°) or PVY tuber necrotic strain (PVYNTN) on potato ‘Kennebec’ plants. The virus isolates were obtained from field ‘Kennebec’ plants and purified by passing through tobacco (for PVX) or potato ‘Jemseg’ (for PVY isolates). Virus-free plantlets of ‘Kennebec’ were sap-inoculated at six-leaf stage with the intended virus isolates or buffer (mock) and kept in the greenhouse. A. Plants at 12 days post-inoculation (dpi). B. Representative leaves from plants shown in A. C. Plants at 35 dpi. D. Representative leaves from plants shown in C. Potato yields (g/plant, mean ± sd, n = 5) from each treatment are shown correspondingly.
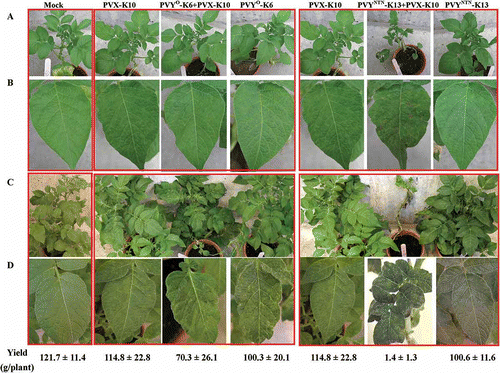
Fig. 4. Systemic symptoms caused by single or double infections with Potato virus X (PVX) and Potato virus Y common strain (PVY°) or PVY tuber necrotic strain (PVYNTN) on tobacco ‘Samsun’ and Physalis floridana plants. The virus isolates were obtained from field ‘Kennebec’ plants and purified by passing through tobacco (for PVX) or potato ‘Jemseg (for PVY isolates). Seedlings of tobacco and P. floridana were sap-inoculated at five-leaf stage with the intended virus isolates or buffer (Mock) and kept in the greenhouse. A. Tobacco plants at 14 days post-inoculation (dpi). B. Tobacco plants at 35 dpi. C. P. floridana plants at 14 dpi. D. P. floridana plants at 45 dpi.