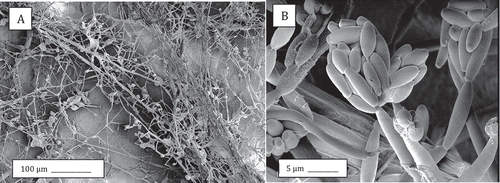
Figures & data
Fig. 1 (Colour online) Hydroponic production of Cannabis sativa. (a) Source (mother) plant for vegetative propagation from which cuttings were obtained; (b) Rooted cuttings in pots filled with clay pellets placed on a propagation bench suspended over a recirculating hydroponic nutrient solution; (c) Plants growing in tubs containing hydroponic nutrient solution. (d) Close-up of healthy plant; (e) Symptoms of browning indicative of root infection; (f) Healthy root system; (g) Colonies of Fusarium oxysporum growing from infected root pieces on PDA; (h) Colony of Pythium sp. on PDA originating from infected roots; (i) Colonies of F. oxysporum recovered from hydroponic nutrient solution.

Fig. 2 (Colour online) Symptoms of Fusarium infection on commercially propagated cannabis plants grown in rockwool blocks. (a) General yellowing of leaves, stunted growth, and necrosis of lower leaves of diseased plant; (b) Stunted growth, curled and necrotic leaves, and death of plant (extreme right); (c) Varying degrees of stunting of two infected plants, with curled and yellowing leaves; (d) The underside of the rockwool block of a diseased plant showing necrotic roots (arrow); (e) Root system of a healthy plant with white roots; (f) Colonies of F. oxysporum emerging from diseased roots plated on PDA; (g) Damping off symptoms on cuttings caused by F. oxysporum; (h) Close-up of stem lesion leading to girdling of rooted cutting in a rockwool block; (i–k) Symptoms on plants artificially inoculated with F. oxysporum, showing yellowing and necrosis of leaves (i), curling and drying of leaves and stunted growth (j) and varying degrees of stunting of plants, curling and necrosis of leaves (k).
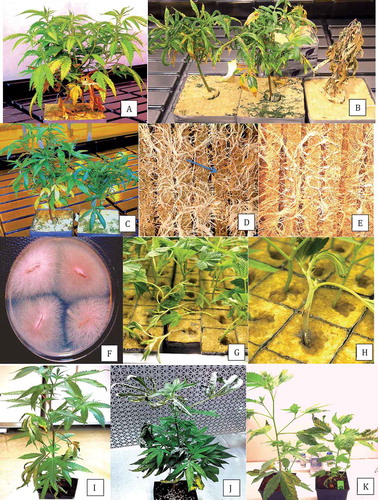
Fig. 3 Phylogenetic analysis of ITS1-5.8S-ITS2 sequences using the neighbour-joining (NJ) method for (a) Pythium dissotocum and (b) Pythium myriotylum originating from cannabis plants compared with isolates from a range of other hosts (GenBank numbers are shown). A bootstrap consensus tree was inferred from 1000 replicates to represent the distance. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates were collapsed.
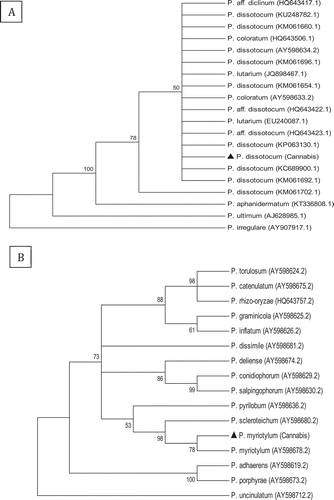
Fig. 4 Phylogenetic analysis of Fusarium oxysporum isolates originating from cannabis plants using (a) ITS1-5.8S-ITS2 sequences and (b) EF-1 sequences compared with isolates from a range of other hosts (GenBank numbers are shown). A bootstrap consensus tree was inferred from 1000 replicates to represent the distance using the neighbour-joining (NJ) method. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates were collapsed. The scale bar indicates the expected number of nucleotide substitutions.
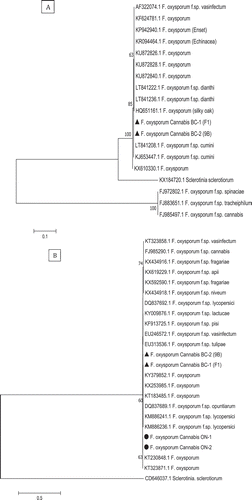
Fig. 5 Phylogenetic analysis of Fusarium solani isolates originating from cannabis plants using ITS1-5.8S-ITS2 sequences compared with isolates from a range of other hosts (GenBank numbers are shown). A bootstrap consensus tree was inferred from 1000 replicates to represent the distance using the neighbour-joining (NJ) method. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates were collapsed. The scale bar indicates the expected number of nucleotide substitutions.
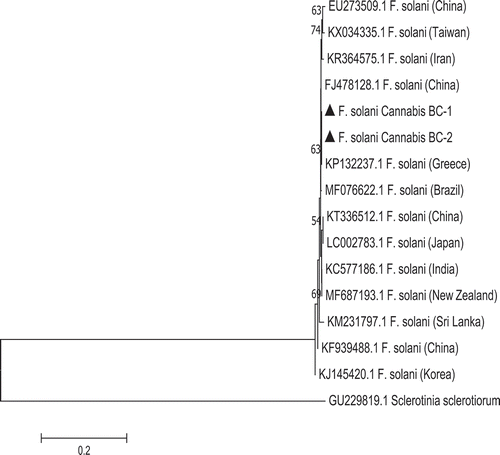
Fig. 6 (Colour online) Pathogenicity studies conducted on rooted cannabis cuttings using isolates of (a) Pythium dissotocum and (b) Fusarium oxysporum conducted in hydroponic solution. In (a), uninoculated control plant is on the right and photo was taken 2 weeks after inoculation; in (b), uninoculated control plant is on the left and photo was taken 3 weeks after inoculation; (c–e) Pathogenicity studies conducted in potting soil comparing control plant (c) to F. oxysporum-inoculated (d) and F. solani-inoculated (e) plants. Photos were taken 21 days after inoculation and show yellowing and necrosis of leaves, and wilting symptoms; (f) Root systems of plants grown in coco fibre showing uninoculated control (left), F. oxysporum-inoculated (middle) and F. solani-inoculated (right); photo was taken 3 weeks after inoculation and shows a significant reduction in root growth compared with the uninoculated control plant; (g) Internal pith and vascular discolouration progressing upward from the crown region in three F. oxysporum-inoculated plants; photo was taken 3 weeks after inoculation.