Figures & data
Fig. 1 Neighbor-Joining tree showing the position of B. subtilis BJ-1 compared with related organisms in a 16S rDNA gene tree. GenBank accession numbers are provided. Numbers at nodes indicate per cent bootstrap support based on 1000 replicates, and only those branches with greater than 50% bootstrap support were labelled. The scale bar at the bottom indicated genetic distance units based on Nei’s genetic distance.
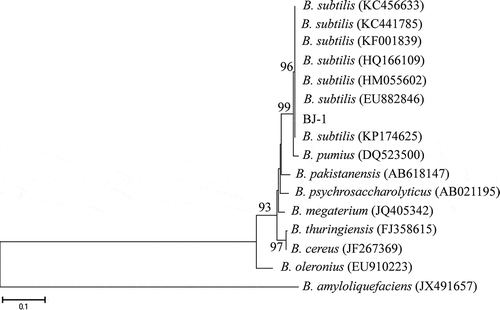
Fig. 2 Identification and expression of antifungal genes from B. subtilis BJ-1. a, PCR amplification of lipopeptide biosynthetic genes from genomic DNA of BJ-1. b, Expression levels of lipopeptide biosynthetic genes were detected using RT-PCR. The housekeeping gene rpoB was amplified as control. Marker: TaKaRa DL1000 (TaKaRa, Dalian, China).
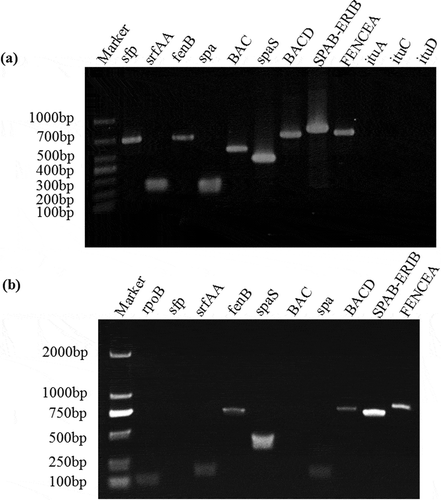
Fig. 3 (Colour online) Effects of culture filtrates of B. subtilis BJ-1 on mycelial morphology, conidial germination and appressorial formation of M. oryzae strain P131. a, Mycelia of M. oryzae without culture filtrate of BJ-1 (CK). b, Mycelia of M. oryzae treated with 0.5% culture filtrate of BJ-1. Arrows indicate abnormal hyphal fragments. c, Conidial germination and appressorial formation without culture filtrate of BJ-1 (CK). d, Malformation of germ tubes with 0.5% culture filtrate of BJ-1. Arrows indicate bulbous germ tubes. Bar = 10 µm.
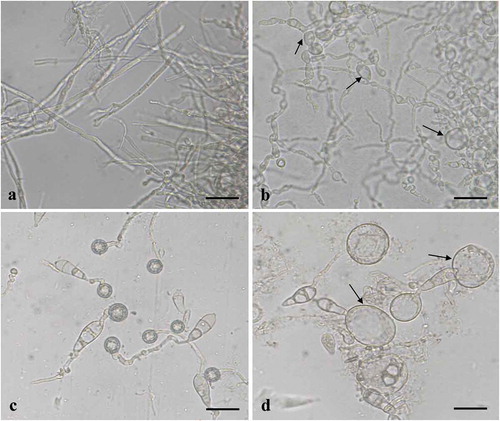
Fig. 4 (Colour online) Control of rice blast disease on detached rice leaves by various concentrations of culture broth (culture filtrate plus bacterial cells), culture filtrates and B. subtilis BJ-1 bacterial cells. a–f, The culture broth of BJ-1 was added to conidial suspensions to obtain final concentrations of 0, 0.5%, 1%, 2.5%, 5% or 10%. g–l, The culture filtrates of BJ-1 were added to conidial suspensions to obtain final concentrations of 0, 0.5%, 1%, 2.5%, 5% or 10%. m–p, BJ-1 bacterial cells obtained by centrifugation were washed three times, resuspended in distilled water and mixed with conidial suspensions to obtain a final concentration of 0, 1 × 107, 1 × 108 or 1 × 109 CFU mL−1. Conidial suspensions of M. oryzae were used at 5 × 105 conidia mL−1.
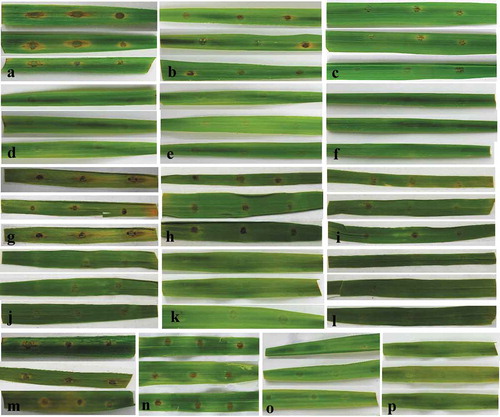
Fig. 5 (Colour online) Control of rice blast in greenhouse by culture broth of B. subtilis BJ-1 on five-leaf stage rice seedlings. a, Rice seedlings without M. oryzae P131 inoculation and BJ-1 treatment served as blank control. b, Rice seedlings only sprayed with conidial suspension (5 × 105 conidia mL−1) served as negative control. c, Rice seedlings sprayed with culture broth of BJ-1 mixed conidial suspension to obtain a final concentration of 10% (v/v). d, Rice seedlings sprayed with tricyclazole (750 μg mL−1) mixed conidial suspension as positive control. e, Rice seeds were soaked with culture broth (10%, v/v) of BJ-1 for 24 h and planted, and then conidial suspension (5 × 105 conidia mL−1) was sprayed onto rice seedlings. f, Seed treatment promoted plant growth of rice. g, Rice plants without any treatments.

Fig. 6 Expression analysis by quantitative RT-PCR of defence-related genes in five-leaf stage rice seedlings after seeds were soaked with culture broth (10%, v/v) of BJ-1. Rice seedlings after seeds were treated with water and LB medium as blank control and negative control, respectively. Expression of OsACTIN gene was used as an internal control. Relative abundance of transcripts of defence-related genes was normalized by comparing with the expression of these genes in rice seedlings after seeds were treated with water (relative transcript level = 1). Data were collected from three independent experiments with three replicates in each treatment. Vertical bars indicated standard error of measurements for three sets of experiments.
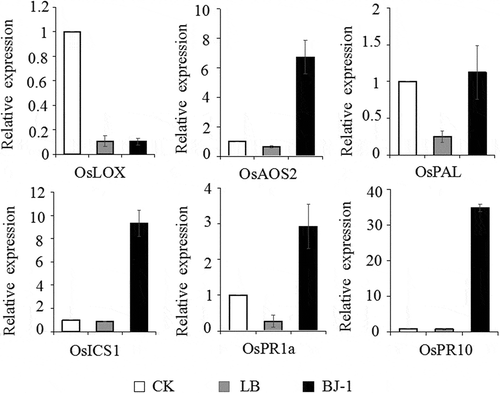
Table 1. Antagonism of B. subtilis BJ-1 toward selected plant pathogenic fungi in dual culture test on PDA plates.
Table 2. Suppression of mycelial growth of M. oryzae in vitro using culture filtrates of B. subtilis BJ-1.
Table 3. Effects of B. subtilis BJ-1 on conidial germination and appressorial formation of M. oryzae on plastic coverslips.
Table 4. Control efficacy of culture broth and culture filtrate of B. subtilis BJ-1 on rice blast development on detached rice leaves.
Table 5. Control efficacy of bacterial suspension of B. subtilis BJ-1 on rice blast development on detached rice leaves.
Table 6. Control efficacy of culture broth of B. subtilis BJ-1 against rice blast in the greenhouse.
