Figures & data
Fig. 1 Sampling of dried cannabis buds for the presence of fungi and colonies recovered on potato dextrose agar with 140 mg L−1 streptomycin sulphate (PDA+S). (a) Dried buds on rack prior to packaging. (b) Close-up of buds that were sampled. (c) Brown discolouration of buds from which Alternaria alternata and Botrytis cinerea were recovered. (d) Mould development on buds from which Penicillium spp. were recovered. (e) The swab method used to recover surface fungi. A cotton swab was gently wiped across the surface of the bud and then streaked across PDA+S. (f) Development of Aspergillus flavus (green) and Penicillium sp. (blue) from a bud streak. (g) Development of Cladosporium westeerdijkieae (brown) and some colonies of Penicillium (green). (h) Development of Botrytis cinerea (white, labelled (a)) and Penicillium citrinum (b). (i) Colonies of Aspergillus niger (a) and Penicilliumspathulatum(b). (j) Colonies of Penicillium pancosmium (a) and P. olsonii (b). (k) Colonies of Fusarium oxysporum (a), A. flavus (b) and Talaromyces pinophilus (c). Colonies of T. radicus (a), P. glabrum (b) and P. chrysogenum (c). All photographs of Petri dishes were taken after 5 days of incubation at 21–24°C

Table 1. Recovery of fungal species from cannabis inflorescences (post-harvest dried buds). The percentage of total Petri dishes which contained at least one colony of the identified species is presented. The closest GenBank sequence match to the identified species is given
Fig. 2 PCR band of ~650 bp size obtained using the universal eukaryotic primers UN-UP18 S42 and UN-LO28 S576B for fungi isolated from cannabis inflorescences. Left panel (a) – L = molecular weight ladder; 1 = Penicillium glabrum; 2 = P. manginii; 3 = Talaromyces pinophilus; 4 = P. olsonii; 5 = P. spathulatum; 6 = P. pancosmium; 7 = P. simplicissimum; 8 = P. terrigenum; C = control (no DNA). Right panel (b) – 1 = Botrytis cinerea; 2 = Alternaria alternata; 3 = Fusarium oxysporum; 4 = F. proliferatum; 5 = F. sporotrichiodes; 6 = F. graminearum; C = Control (no DNA); L = molecular weight ladder
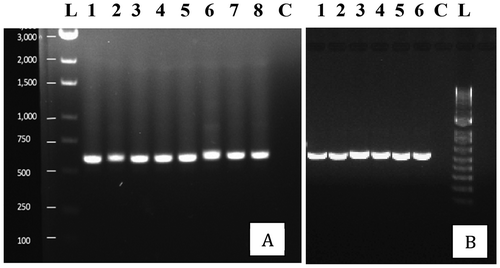
Fig. 3 Disease symptoms on greenhouse-grown cannabis plants and recovery of pathogens from diseased inflorescences. (a) Yellowing of lower leaves due to crown infection by Fusarium oxysporum. (b) Infected inflorescences (buds) from which F. oxysporum was recovered. (c) Close-up of diseased inflorescence showing mycelial growth of F. oxysporum under greenhouse conditions. (d) Symptoms of bud rot caused by Botrytis cinerea showing necrotic tissues and mycelial growth. (e) Mycelial growth of Fusarium sporotrichioides on harvested bud in the drying room. (f) Mycelial growth on freshly harvested inflorescences which were incubated under moist conditions in the laboratory for 5 days. The bud on the left has F. sporotrichioides and the one on the right has F. proliferatum. (g) Development of F. oxysporum from a bud streak. Blue-green colonies are those of P. spathulatum. (h) Development of F. proliferatum (pink-orange colonies) from a bud streak. The sparse white mycelium also growing on edges of the plate is B. cinerea. (i) Colony of F. sporotrichioides. (j) Colony of F. oxysporum. (k) Colony of B. cinerea. (l) Colony of Alternaria chlamydosporigena. Colonies shown in (i–k) were photographed after 10 days of growth on PDA at 21–24°C
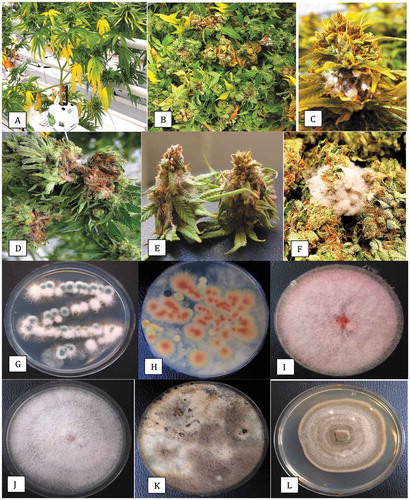
Fig. 4 Inoculation of detached inflorescences of cannabis with a mycelial plug of each of four pathogens. Buds were incubated in a moist chamber for 5 days. (a) Botrytis cinerea. (b) Fusarium oxysporum. (c) F. sporotrichioides. (d) F. graminearum. The relative growth rates of the pathogens can be seen colonizing the tissues

Fig. 5 The effect of temperature on growth rate and colony morphology of three pathogens recovered from cannabis inflorescences. Photographs were taken after 7 days of growth at each of the temperatures, as follows. (a) Botrytis cinerea at 5, 10, 15, 20, 25, 30°C. (b) Fusarium sporotrichioides at 5, 10, 15, 20, 25, 30°C. (c) Fusarium oxysporum at 10, 15, 20, 25, 30, 35°C
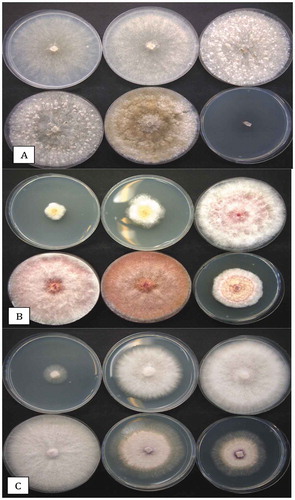
Fig. 6 Colony morphologies of the more distinct fungi that were recovered from cannabis inflorescences. All cultures were grown on PDA at 21–24°C. (a) Penicillium copticola. (b) Penicillium pancosmium. (c) Talaromyces radicus. (d, e) Cultures made using cotton swabs to streak spores across the surface of PDA plates. Cultures were grown for 7 days. (d) Top view of colony features of Talaromyces pinophilus (upper left), P. pancosmium (upper right), P. sclerotiorum (lower left), P. glabrum (lower right). (e) Bottom view of the same cultures shown in (d) with characteristic pigment production
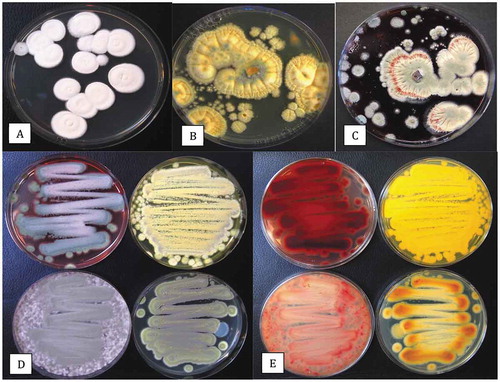
Fig. 7 Colonies of a wide range of fungi growing on Petri dishes containing potato dextrose agar+140 mg L−1 of streptomycin sulphate. Lids of the dishes were removed and the dishes were exposed to the ambient environment in (a) a greenhouse growing facility and (b) an indoor growing facility for 60 min. Dishes were incubated for 7 days at 21–24°C after which the photographs were taken. In (a), the brown colonies are Cladosporium westeerdijkieae, the blue-green colonies are Penicillium olsonii, and the white colonies are P. spathulatum. In (b), the yellow colonies are Aspergillus ochraceus and the remainder are Penicillium spp., including P. olsonii, P. simplicissimum and P. glabrum.
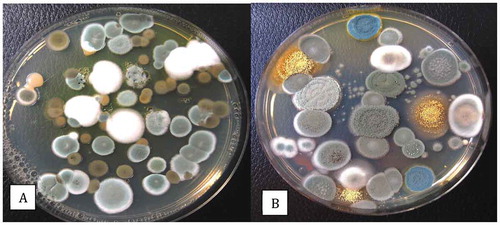
Fig. 8 Recovery of Penicillium spp. on PDA+S following swab samples taken from cannabis buds at different stages of processing. The swabs were obtained from harvested (non-dried) buds, buds after completion of mechanical trimming, and from trim fragments. The total colony-forming units are shown in (a) and the appearance of the dishes from Trial 1 is shown in (b). Data are from 10 replicate dishes in each of two trials
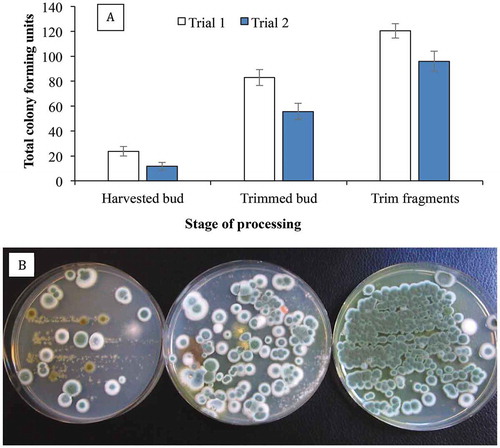
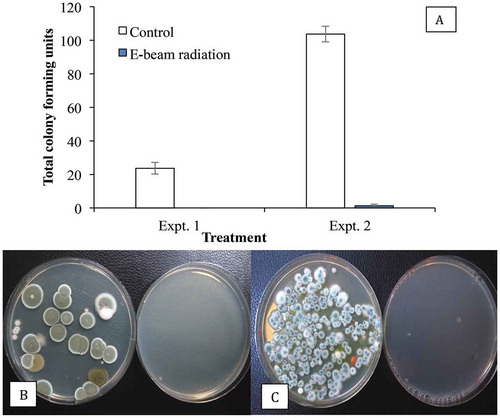
Fig. 9 Recovery of Penicillium spp. on PDA+S following swab samples taken from cannabis buds after E-beam radiation compared with non-treated (control) buds. The total colony-forming units are shown in (a) and the appearance of the dishes from Expt. 1 is shown in (b) and from Expt. 2 in (c). Data are from 10 replicate dishes in each trial. In both photos, control dishes are on the left and treated dishes on the right