Figures & data
Table 1. Demographics and characteristics of all randomized participants at screening (numbers and percentages; mean values with their standard errors).
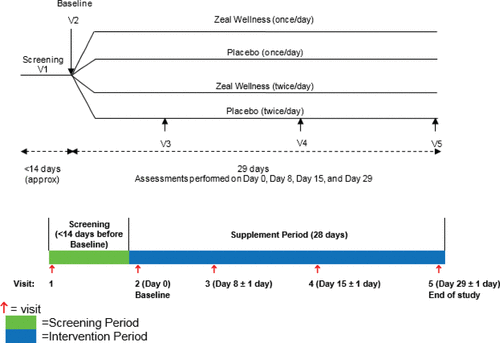
Figure 2. Disposition of study participants. A total of 121 potential participants were screened and 99 eligible participants were enrolled in the study (with 25 participants in each study group, except for 1-dose Zeal Wellness, which had 24). Ninety-four participants completed the study. Fifteen participants were removed from the per protocol (PP) analysis; nine participants had missing Profile of Mood States, two participants were noncompliant, one participant had low compliance, two participants withdrew from the study because of an adverse event, and one participant requested to be removed from the study. ITT = intent-to-treat.
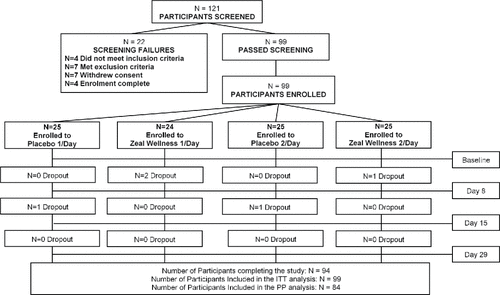
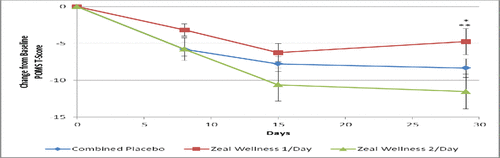
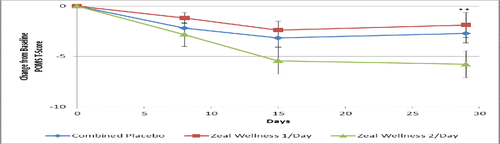
Figure 3. Total mood disturbance score results at days 0, 8, 15, and 29 for participants receiving supplementation with Zeal Wellness 1/day, Zeal Wellness 2/day, and placebo (N = 99). POMS = Profile of Mood States.