Figures & data
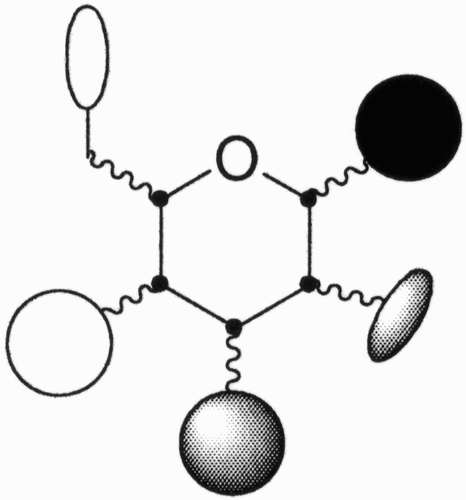
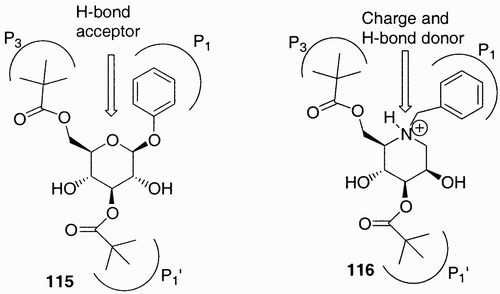
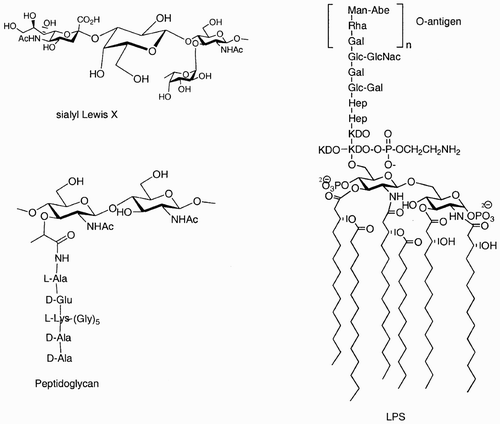
Figure 1: Examples of how nature exploits carbohydrates as scaffolds that link other sugars, lipids, peptides, and phosphates in well‐defined positions and orientations.
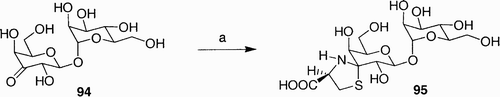
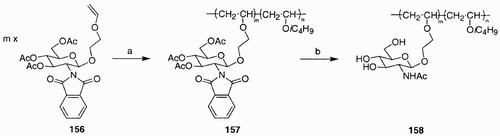
Scheme 1: Reagents and conditions: a) i. n‐Bu2SnO, MeOH, Δ; ii. BnBr, CsF, DMF, 25°C, 14 h, 93%; b) DAST, CH2Cl2, 40°C, 4 h, 63% (α/β, 2:3); c) Cp2ZrCl2, AgClO4, HOCH2CO2Et, 4 Å mol. sieves, PhH, 25°C, 4 h, 55% (α/β, 2:3); d) Ph3P, THF, H2O, 65°C, 4 h, 85%; e) LiOH, THF/H2O (8:1), 25°C, 4 h; f) 1H‐pyrazole‐1‐carboxamidine · HCl, i‐Pr2NEt, DMF, 25°C, 16 h, 80% over two steps.
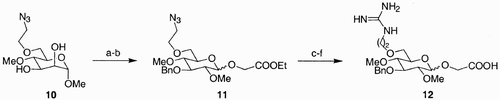
Scheme 2: Reagents and conditions: a) HATU, DIPEA, DMF, rt 100%; b) 0.5 M i‐PrNCO/DMF, Cu(I)Cl, rt 100%; c) 20% piperidine/DMF, rt d) 4‐NO2C6H4COOH, HATU, DIPEA, DMF, rt e) 10% TFA/1,2‐dichloroethane, rt 100%.
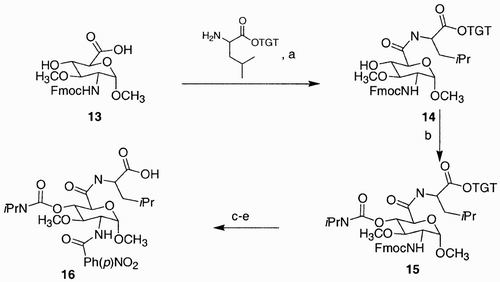
Scheme 3: Reagents and conditions: a) MeOH, AcCl, 100%; b) PhCH(OMe)2, p‐TsOH, DMF, 70°C, 69%; c) DMSO, (CF3CO)2O, Et3N, CH2Cl2, −78°C, 75%; d) L‐selectride, THF, −78°C, 60%; e) N2H4, 130°C, 88%; f) 1.1 eq. PrI, K2CO3, MeCN, reflux, 63%; g) H2O2, Na2WO4, MeOH, H2O, 46% then LiAlH4, THF, 0–50 °C, 28%.
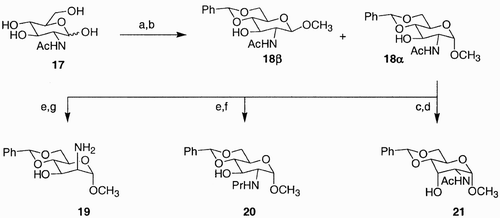
Scheme 4: Reagents and conditions: a) TBPSCl, imidazole, DMF, 100%; b) i. Bu2SnO, toluene, benzene, reflux; ii. PMBCl, Bu4NI, DMF, 60°C, 49%; c) ClCH2COCl, Et3N, CH2Cl2, −20°C to rt 52%; d) levulinic acid, DCC, DMAP, CH2Cl2, 83%, e) i. HO(CH2)5CO2Me, NIS, TMSOTf, 4 Å molecular sieves, CH3CN, −20°C to rt; ii. HgBr2, toluene, CH3NO2, 60°C, 85%; f) NH2‐NH2 AcOH, THF/MeOH, 10:1, 90%; g) NaHCO3, MeOH/H2O, 5:1, 60°C, 99%; h) HF‐pyridine, AcOH/THF, 1:4, 98%; i) CF3COOH, CH2Cl2, −20°C, 97%.
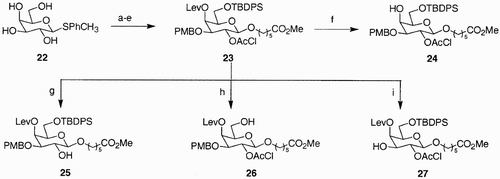
Scheme 5: Reagents and conditions: a) TBDMSCl, Imidazole, CH2Cl2, 94%; b) AllBr, NaH, DMF, 70%; c) LiAlH4‐AlCl3, CH2Cl2, Et2O, 82%; d) succinic anhydride, pyridine, DMAP; e) NH2‐PS/DV, HOBt, HBTU, DIPEA, DMF.
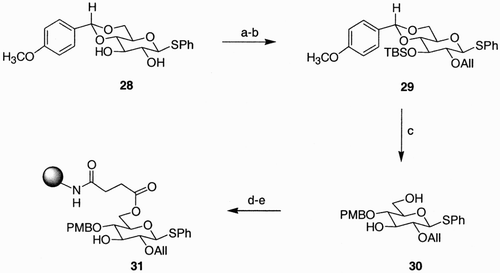
Scheme 6: Reagents and conditions: a) TBAF, THF; b) DDQ, CH2Cl2, H2O (10 vol%); c) i. Bu3P, DMF; ii. Et3N, DMF, H2O; d) [Pd2(dba)3], pTsOH, DMF; all yields are quantitative.
![Scheme 6: Reagents and conditions: a) TBAF, THF; b) DDQ, CH2Cl2, H2O (10 vol%); c) i. Bu3P, DMF; ii. Et3N, DMF, H2O; d) [Pd2(dba)3], pTsOH, DMF; all yields are quantitative.](/cms/asset/6a4b407e-ab21-4454-a349-0d4d60bdf777/lcar_a_173266_o_sch0006g.gif)
Scheme 7: Reagents and conditions: a) LiOH, THF, H2O, quant.; b) TOTU, HOBt, DIPEA, DMF, aminomethylpolystyrene; c) Ac2O, pyridine, dioxane, 98%; d) NH2‐NH2 H2O, DMF, e) KOtBu, DMF; f) MeBr, DMF; g) TBAF, THF; h) FC6H4NCO, DMAP, dioxane; i) PPTS, MeOH, dioxane; j) Steglich esterification at position 4; k) p‐TsOH, [Pd(PPh3)]4, DME, dioxane; l) NBS, EtOH, DTBP, CH2Cl2; m) Et4NBr, cyclohexene, CH2Cl2, 79% yield.
![Scheme 7: Reagents and conditions: a) LiOH, THF, H2O, quant.; b) TOTU, HOBt, DIPEA, DMF, aminomethylpolystyrene; c) Ac2O, pyridine, dioxane, 98%; d) NH2‐NH2 H2O, DMF, e) KOtBu, DMF; f) MeBr, DMF; g) TBAF, THF; h) FC6H4NCO, DMAP, dioxane; i) PPTS, MeOH, dioxane; j) Steglich esterification at position 4; k) p‐TsOH, [Pd(PPh3)]4, DME, dioxane; l) NBS, EtOH, DTBP, CH2Cl2; m) Et4NBr, cyclohexene, CH2Cl2, 79% yield.](/cms/asset/89939021-7bf3-4e10-84b9-9a2e3a926807/lcar_a_173266_o_sch0007g.gif)
Scheme 8: Reagents and conditions: a) i. O3, MeOH, THF, −70°C; ii. NaBH4; b) PPh3, NBS, DMF, 50°C, c) C3H7NH2, DMF, 70°C, d) (Boc)2O, NEt3, CH2Cl2, e) TFA/H2O, 3:1.
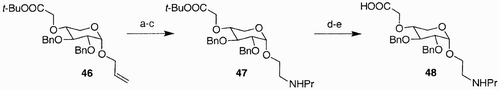
Scheme 9: Reagents and conditions: a) 5 steps, 32%; b) Tf2O, Py, DCM; c) CsO2CCF3, butanone; d) MsCl, DMAP, pyridine, 100%; e) NaN3, DMF, 70%; HCl, MeOH, 78%; f) HCl, MeOH, rt, 73%; g) MeI/CH3CN (1:1), Ag2O, 80°C, 99%; h) H2, Pd/C 10%, EtOAc, i) i. ClC6H4NCO, CH2Cl2, rt, 16 h; ii. AMP's, rt, 16 h; j) PhC3H6NH2, MeOH, 60°C, 24 h, 83%.
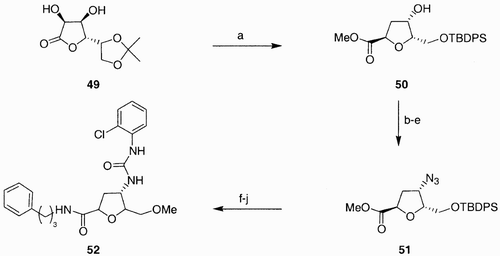
Scheme 10: Reagents and conditions: a) BOP, DIPEA, 16 h; b) Bn‐N˭C˭O, Et3N, 16 h; c) i. Me3P, THF, 1 h, then H2O/dioxane 2 h; ii. Ph2NCOCl, DIPEA, 16 h; d) 56 (5 mol%), CH2Cl2, reflux, 16 h, 98% overall yield.
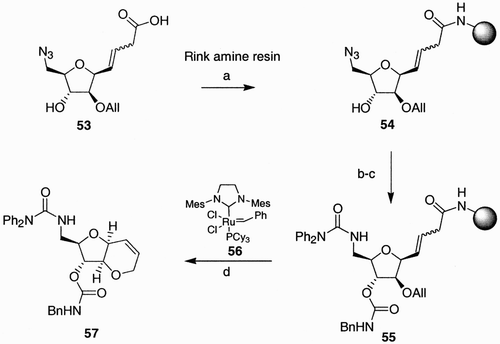
Scheme 11: Reagents and conditions: a) I2, THF; b) Zn, AcOH, EtOH/Et2O, 1:1; c) DMSO/Ac2O, 2:1; d) CH2˭CHCH2MgBr, THF; e) I2, THF, 0°C; f) I2, CH2Cl2, rt; g) NaN3, Bu4NI, DMF; h) NaClO2, NaH2PO4, CH3CN.
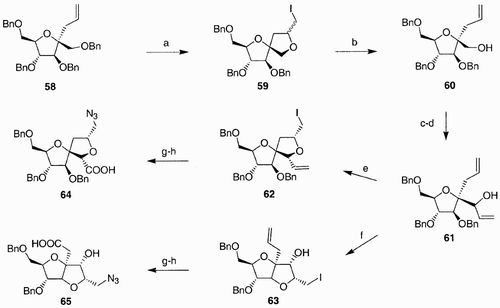
Scheme 12: Reagents and conditions: a) HOBT, HBTU, DIPEA, DMF; b) i. Bu3P, DIC, Fmoc‐Asp(OtBu)‐OH; ii. piperidine 20% in DMF; iii. 1% TFA in CH2Cl2; c) i. 0.5 mM in DMF, HBTU, HOBt, DIPEA; ii. 95% TFA, 2.5% TIS 2.5% H2O.
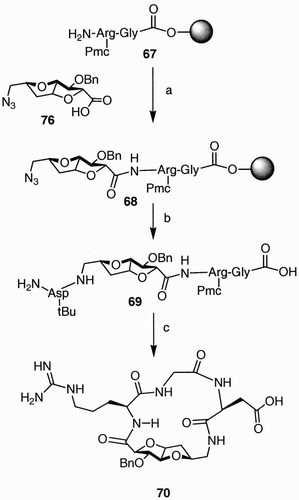
Scheme 13: Reagents and conditions: a) BnNH2 (neat), 48 h; b) H2, 10% Pd/C, EtOAc; c) Z‐NHCH(CH3)CO2H, PyBOP, Et3N, DMF, rt, 14 h, 92% from 74; d) K2CO3, MeOH/H2O (10:1), rt, 48 h; e) H2, 10% Pd/C, EtOH/EtOAc (1.5:1); f) DPPA, Et3N, DMF, 0°C to rt, 14 h.
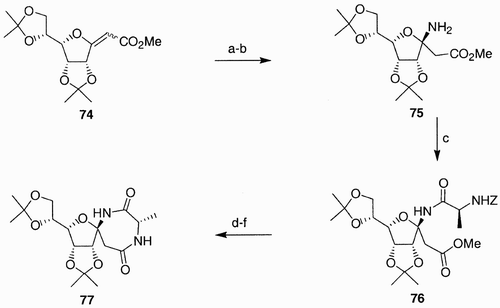
Scheme 15: Reagents and conditions: a) i. BrHC˭CHCH3, n‐BuLi, THF, −78°C; ii. AllBr, HMPA, −78°C to rt; b) ref. [51]; c) 2‐butyn‐1‐ol, K‐10, mol. sieves, CH2Cl2; d) Ru‐catalyst (5–7 mol%), toluene, 60°C.
![Scheme 15: Reagents and conditions: a) i. BrHC˭CHCH3, n‐BuLi, THF, −78°C; ii. AllBr, HMPA, −78°C to rt; b) ref. [51]; c) 2‐butyn‐1‐ol, K‐10, mol. sieves, CH2Cl2; d) Ru‐catalyst (5–7 mol%), toluene, 60°C.](/cms/asset/8d3e6c02-7be5-400a-ac6f-9391fb9fcd98/lcar_a_173266_o_sch0015g.gif)
Scheme 17: Reagents and conditions: a) NH2OMe, THF, EtOH; b) LiAlH4, THF; c) FmocCl, DIPEA, CH3CN; d) I2, DME; e) NaOH 1 M, CH3CN; f) NaOH 3 M, EtOH reflux; g) FmocCl, DIPEA, CH3CN; h) Jones oxidation.
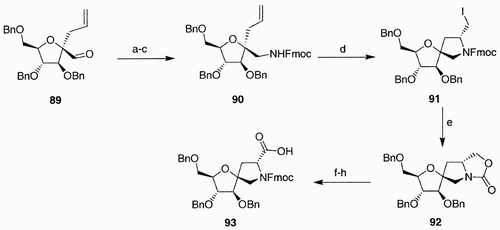
Scheme 22: Reagents and conditions: a) TBAF, THF; b) MsCl, Py, CH2Cl2; c) NaN3, DMF; d) LiOH, MeOH/H2O/THF; e) HBTU, HOBT, DIPEA, DMF, Xaa.
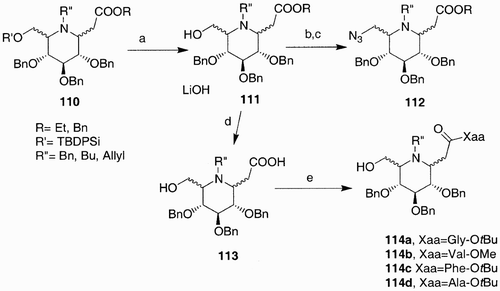
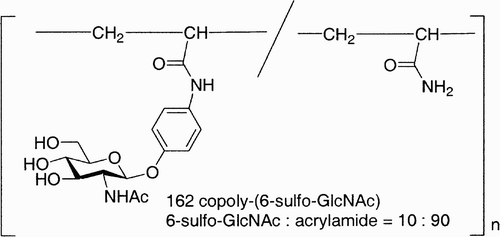
Figure 16: 117, Hyaluronic acid; 118, 3,3′‐dithiobis(propanoic dihydrazide); 119, 1,3,5‐benzene(tricarboxylic trihydrazide); 120, poly(ethylen glycol)‐diamine tetrapropanoic tetrahydrazide.
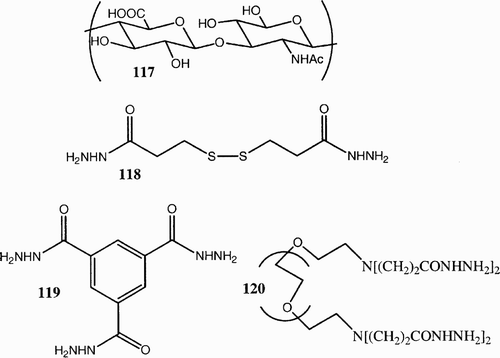
Scheme 23: Polyacetylation of methyl α‐d‐mannopyranoside (123) with 1,4‐bis(2‐formyl‐phenoxy)butane (124).
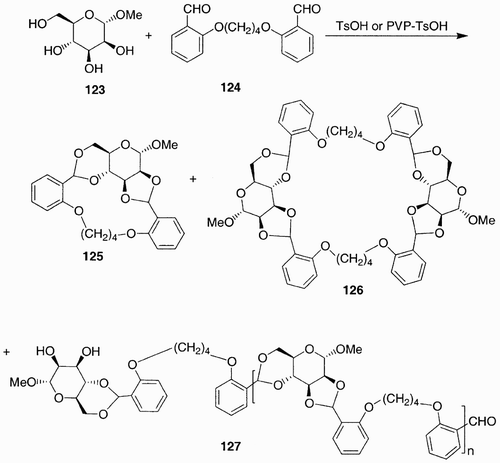
Scheme 24: Examples of PHPAs form the polymerization of dimethyl galactarate (128) and d‐glucarate 1,4‐lactone (135) with diamines.
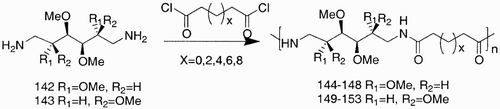
Scheme 26: Reagents and conditions: a) N‐methyl‐2‐pyrrolidinone, ethyldiisopropylamine, 15 days, 25°C.