Figures & data
Figure 1. Patent expirations for major biologics. Biosimilars are under development for the biologics (originators) which are going off patents. The exclusivity period differs in the US and EU for the same molecules, hence represented separately but color coded to make it easier for the comparison. Brand names, scientific names and the company holding the patent are provided for each.
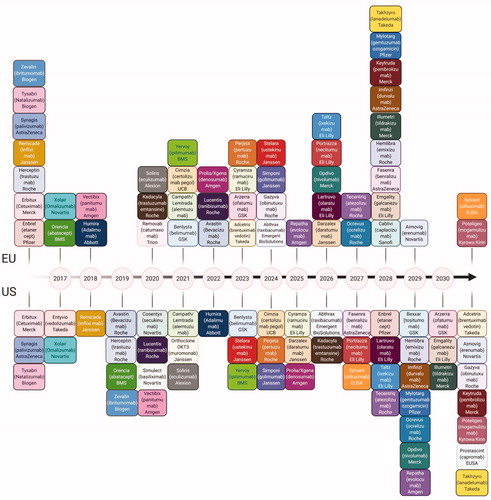
Figure 2. Market Trends in Biopharmaceuticals. (A) Top selling biopharmaceuticals from 2018 globally. (B) As patents for top selling biologics are expiring, an increasing amount of biosimilars and biobetters are reaching developmental milestones. (C) The number of biotherapeutics in phase 1 by category demonstrating the developmental superiority of mAbs in the current biopharmaceutical market. (D) Number of compounds in development according to the therapeutic category.
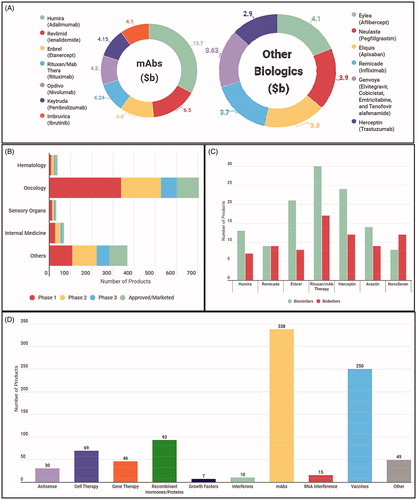
Figure 3. Comparison of Originator and Biosimilar Development Pathways. (A) Development pathway of new originator molecules. This is the likely pathway to be followed by the biobetters as well. (B) Abbreviated pathway followed for the biosimilars. (C) Physicochemical characteristics that require monitoring and characterization, some of them for, the critical quality attributes. (D) Process related impurities that require removal from the final batches. (E) Other factors to consider that may cause little variations hence, extremely important while determining similarity.
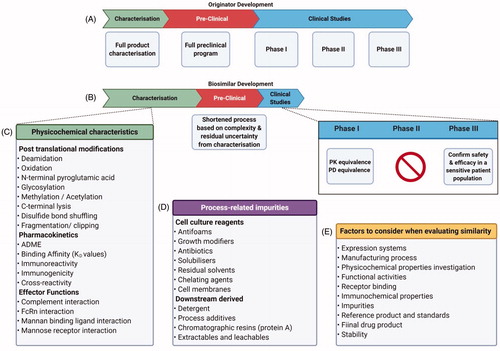
Figure 4. CMC activities over an 18-month period for the development of monoclonal antibodies Analytical Methods Development is carried almost throughout the 10 months while upstream process development leads to downstream process development with some important overlap. Formulation development and drug manufacturability become a very important consideration early on, the details of individual steps are entailed in (Created with Biorender.com).
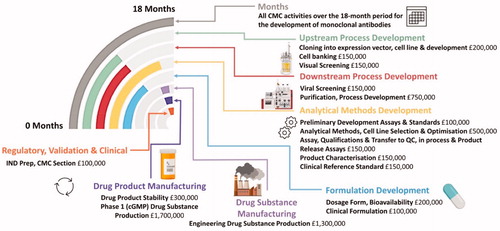
Table 1. Stages and timescales involved in the production of a monoclonal antibody biosimilars.
Table 2. ICH guidance documents covering the testing & characterization of biological products.
Table 3. Selected examples of top selling biologics compared to other leading biologics demonstrating the impact mAbs on global market share (2018).
Figure 5. Downstream signaling of TNF alpha. TNF alpha has impact on cartilage matrix, endothelial cells, synoviocytes, macrophages/monocytes and prostanoids; playing a key role the pathogenesis of rheumatoid arthritis (Created with Biorender.com).
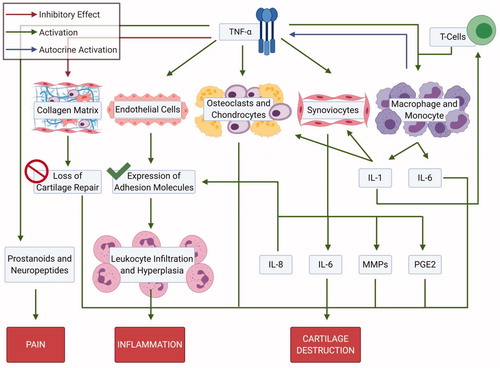
Figure 6. Pathophysiology of rheumatoid arthritis. (A) Locations of impacted areas in Rheumatoid Arthritis. (B) A healthy synovial joint and the impact of RA. In a healthy joint (left), there is no swelling in the synovial joint capsule, cartilage and bone are intact. Whereas, in RA (right), there is swelling, cartilage and bone erosion. (C) Mechanism of RA within the joint capsule. TNF alpha impacts synoviocytes, macrophages/monocytes and osteoclasts; inititating RA pathogenesis. Synoviocytes line the capsule and T-cells infiltrate the synovial membrane, initiating inflammation via TNF alpha. Joint degradation occurs via recruitment of macrophages and secretion of inflammatory cytokines. Bone erosion occurs by osteoclasts and inhibition of collagen secretion by synoviocytes (Created with Biorender.com).
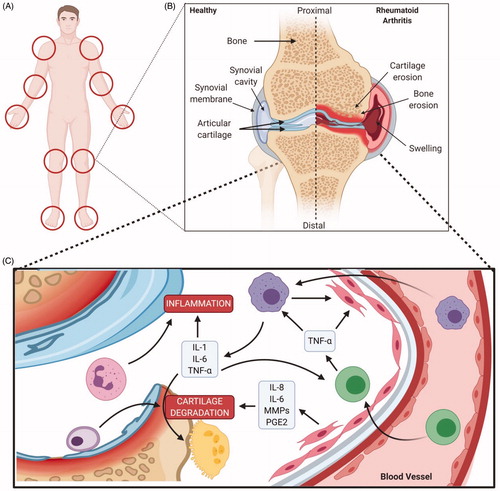
Table 4. FDA approved Anti-TNFα biologics and ts-DMARDS.
Figure 7. Structural representation of selected FDA-approved anti-TNFα biologics. Infliximab, Adalimumab and Golimumab are complete antibodies while Etanercept is a fusion protein whereas Certolizumab pegol has a humanized Fab region, hence devoid of effector functions (Created with Biorender.com).
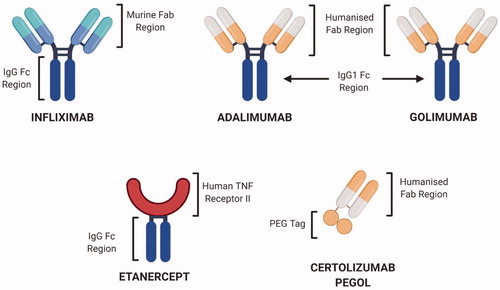
Table 5. TNF inhibitor biosimilars approved worldwide [Citation81,Citation140–144].
Figure 8. Glycan variation shown in biotherapeutics. Variation occurring through development can be accounted to distinctive glycan machinery present in the chosen expression system. Adapted from several sources [Citation154–157].
![Figure 8. Glycan variation shown in biotherapeutics. Variation occurring through development can be accounted to distinctive glycan machinery present in the chosen expression system. Adapted from several sources [Citation154–157].](/cms/asset/8261e4ac-fa19-4b74-bd13-c1825b922b86/ibty_a_1830746_f0008_c.jpg)
Figure 9. Glycan analysis. Flowchart depicting sample preparation for glycan analysis, which typically involve lengthy denaturation and labeling steps to acquire sample for analysis.
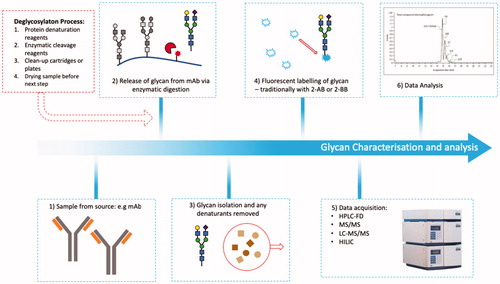
Table 6. Analytical procedures for the analysis of mAb critical quality attributes.
Table 7. TNF inhibitor biosimilars in development with respective developmental stages.
Table 8. Intended copies launched worldwide.
