Figures & data
Figure 1 The cC monomeric structure with selected residues Ile66, Ile108 in the hydrophobic core, and Val55 in Loop 1.
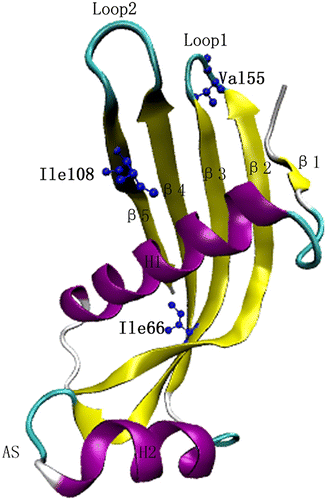
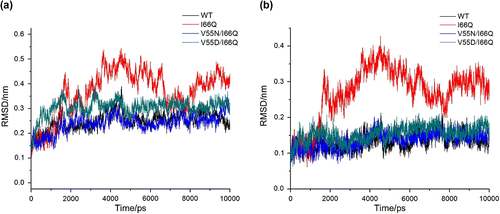
Figure 2 Time dependence of the RMSD from the total amino acid of cC for the Cα (A) and the hydrophobic core of cC for the backbone (B) in the 10 ns MD simulation.