Figures & data
Figure 1 Secondary structure topology of CHASE domain of AtHK1 showing 11 β-sheets and 8 α-helices structures (A) and OsHK3b (B) showing 14 β-sheets and 8 α-helices.
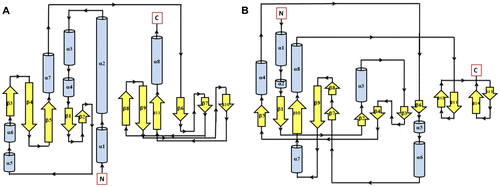
Figure 2 Cartoon view diagram of the secondary structure of CHASE domain of AtHK1 showing the arrangement of 11 β-sheets and 8 α-helices structures (A) and OsHK3b showing 14 β-sheets and 8 α-helices (B). The highlighted residues were found conserved in various sensory domains. The residues are numbered according to their respective position in the complete sequence of AtHK1 and OsHK3b.
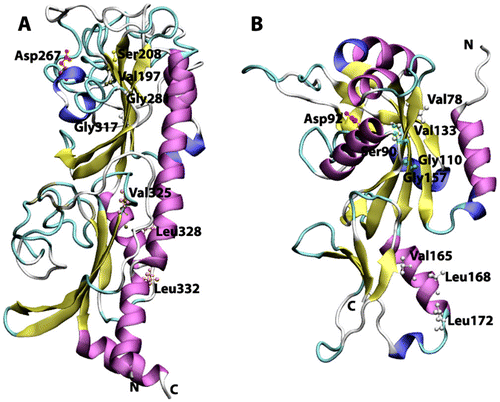
Figure 3 Secondary structure topology of TD domain of showing presence of 7 β-sheets and 7 α-helices in AtHK1 (A) and 5 β-sheets and 7 α-helices in OsHK3b (B).
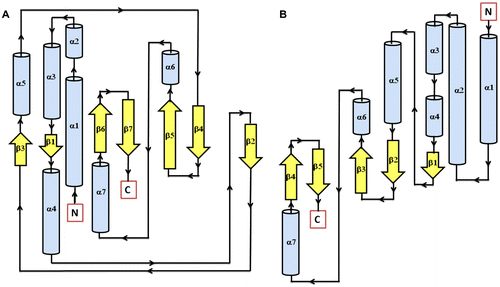
Table 1. Hydrogen bonds between ADP and modeled TD of AtHK1. Hydrogen bond distances between the residues were calculated using HBPLUS.
Table 2. Hydrogen bonds between ADP and modeled TD of OsHK3b. Hydrogen bond distances between the residues were calculated using HBPLUS.
Figure 4 Cartoon view diagram of the secondary structure of TD domain showing the arrangement of 7 β-sheets and 7 α-helices in AtHK1 (A) and of 5 β-sheets and 7 α-helices in OsHK3b (B) with ADP and Mg along with the marked residues which were observed to form hydrogen bond with ADP. The residues are numbered according to their respective position in the complete sequence of AtHK1 and OsHK3b.
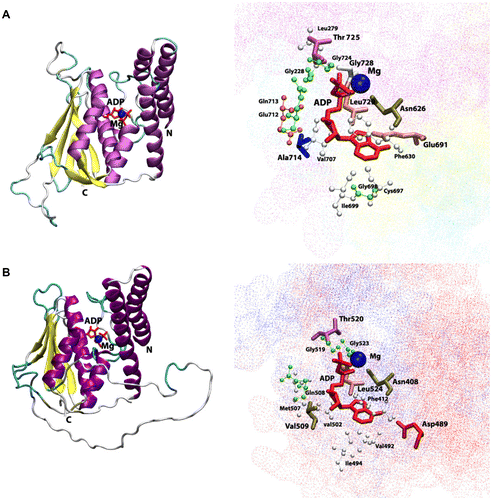
Figure 5 Secondary structure topology of RD showing the presence of 5 β-sheets and 6 α-helices in both AtHK1 (A) and OsHK3b (B).
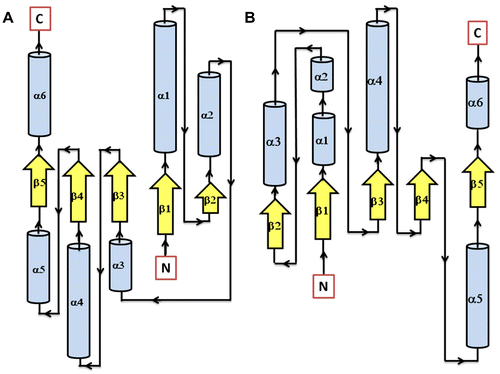
Figure 6 Cartoon view diagram of the secondary structure of RD showing the presence of 5 β-sheets and 6 α-helices arrangements in both AtHK1 (A) and OsHK3b (B) showing conserved residues. The residues are numbered according to their respective position in the complete sequence of AtHK1 and OsHK3b.
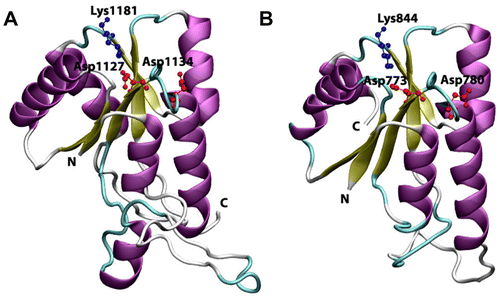
Figure 7 Secondary structure topology of HPt protein showing the presence of 7 α-helices in Arabidopsis (AtHPt1) (A) and 6 α-helices in O. sativa (OsHPt2) (B).
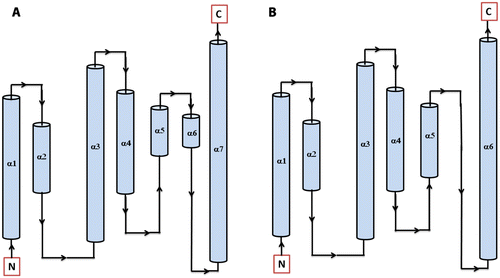
Figure 8 Cartoon view diagrams of the secondary structure arrangements of 7 α-helices in Arabidopsis (AtHPt1) (A) and 6 helices in O. sativa (OsHPt2) (B) with the conserved His residue. The residues are numbered according to their respective position in the complete sequence of AtHPt1 and OsHPt2.
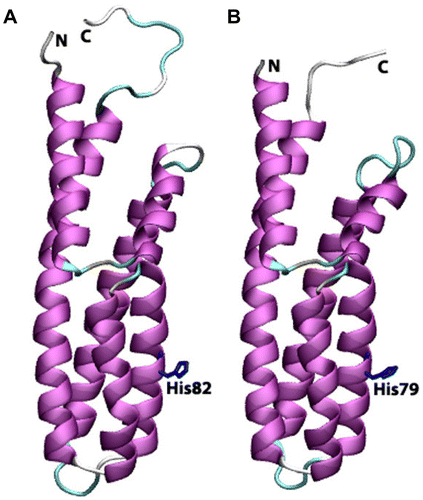
Figure 9 Cartoon view diagram of the docking of RD with HPt protein in Arabidopsis (A) and O. sativa (B) showing residues which plays role in protein–protein interactions. The residues are numbered according to their respective position in the complete sequence of AtHK1, OsHK3b, AtHPt1, and OsHPt2.
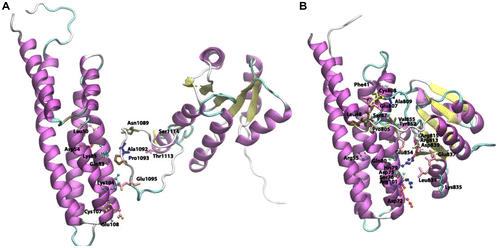
Table 3. Comparative analysis of the secondary structure elements in AtHK1, OsHK3b, AtHPt1, and OsHPt2 proteins.
