Figures & data
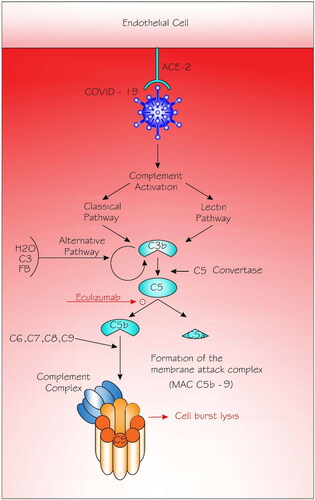
Figure 1. Complement activation in COVID-19 infection.
Angiotensin-converting enzyme (ACE2) plays a crucial role in the initiation of COVID-19 infection by attaching to the endothelial cells and hence having the domino effect on the infection and defense processes including the complement system. The classical pathway is triggered by antibody or by direct binding of complement component C1q (not shown) to the pathogen surface; the lectin pathway, which is triggered by mannan-binding lectin binds some encapsulated bacteria; and the alternative pathway is triggered directly on pathogen surfaces. Classical and lectin pathways are activated resulting in the formation of the C3b. The alternative pathway is activated by hydrolysis (tick-over) of C3 that forms C3 (H2O). The latter binds to factor B (fB), which is eventually cleaved by factor D (not shown) to form the alternative pathway fluid-phase initiation C3 convertase. The C3 convertases cleave C3 into C3a and C3b. Eventually, C3b contributes to the configuration of the C5 convertases that cleave C5, leading to the production of C5a and C5b. It is C5b that instigates the terminal events of complement activation, resulting in the formation of the membrane-attack complex (MAC or C5b-9 complex). Eculizumab (a drug, and not part of the complement cascade) inhibits the cleavage of C5 into C5a and C5b and hence prevents the generation of the terminal complement complex C5b-9.