Figures & data
Figure 1. PRISMA flow diagram. A total of 114 studies were obtained with the search strategy of which finally 24 were included.
Figure 2. Forest plot of mixed treatment comparison estimates for the incidence of AMS. Acetazolamide 375 mg twice daily, dexamethasone, ibuprofen, acetazolamide 125 mg twice daily, combined acetazolamide with Ginkgo biloba and acetazolamide 250 mg twice daily were observed with significantly lower incidence of AMS compared to placebo.
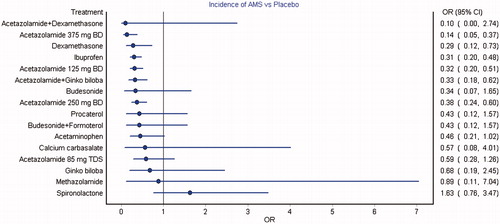
Table 1. Mixed treatment comparison pooled estimates {odds ratio [95% confidence interval]} for the key comparisons for incidence of AMS.
Figure 3. Forest plot of mixed treatment comparison estimates for incidence of severe AMS. Acetazolamide at 125, 250 and 375 mg twice daily, dexamethasone and combined acetazolamide with Ginkgo biloba showed a significant reduction in the incidence of severe AMS compared to placebo.
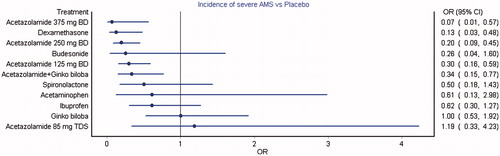
Table 2. Grading the evidence for key interventions compared to placebo for the incidence of AMS.
