Figures & data
Table 1. The role of viral regulatory proteins in HAND.
Figure 1. Schematic representation of mechanisms of HIV neurotoxicity. The Gp41, Gp120, Tat, Vpr, Vpu and Nef viral proteins, that circulate in the blood produce enhanced ROS production, results in alteration of BBB. Different process occurs during the neurotoxicity like enhanced oxidation of DNA nucleic bases, genomic instability, aggregation of ceramide, stimulation of A-type transient outward K + currents by Kv channels, and production of proinflammatory cytokines.
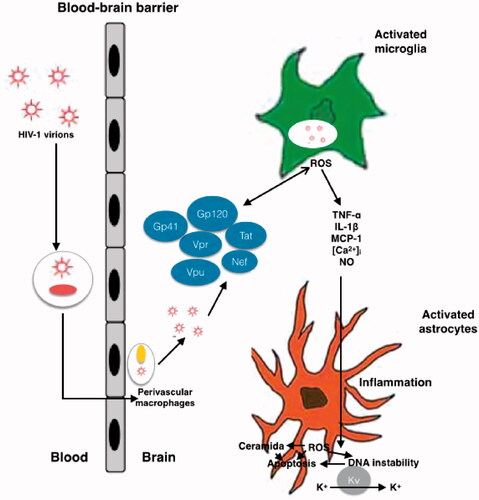
Table 2. Typification by cognitive impairment and recommendations for Monitoring Patients With HAND.
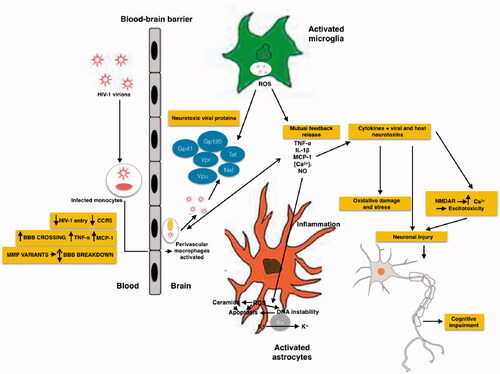
Figure 2. Schematic representation of the effects of HIV and its viral proteins on the cells of the CNS of HAND patients. HIV enters into the brain, especially perivascular space (the main site for viral replication), within infected macrophages or as free virions or viral particles. HIV infects and activates macrophages, astrocytes and microglia in the perivascular space, the main site for viral replication. Macrophages, and astrocytes and activated microglia contribute to the release of proinflammatory cytokines and chemokines, which provoke additional influx of immune cells and mediate neuronal damage. Conversely, some inflammatory mediators can also promote neuronal survival. HIV induces activated astrocytes which secrete proinflammatory cytokines, chemokines and glutamate. Together with viral proteins and HIV-induced chemokines, these substances overstimulate NMDA receptors, causing excitotoxicity. All the events caused by HIV proteins, excitotoxicity, and inflammation lead to axonal injury and neuronal apoptosis.
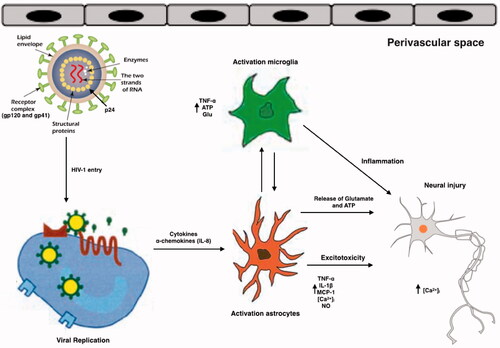
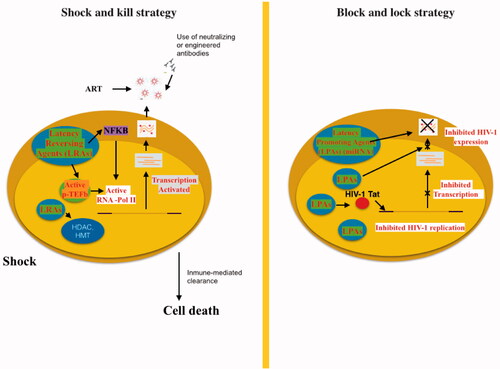
Figure 3. “Shock and kill” strategy involves activating viral replication to eliminate reservoirs and to target latently-infected cells. LRAs have an important role in the reactivate transcription for the “shock”. LRAs promote chromatin decompaction and ARN pol II recruitment to induce virus transcription. ART is maintained during this phase to clear reservoir without virus propagation in other cells. The “kill” can be enhanced by stimulation of the cell-mediated immune response or by using neutralising and/or engineered antibodies. The “block and lock” strategy relates on the induction of a state of deep-latency to prevent HIV-1 transcription. LPA inhibit various step of virus replication, transcription by Tat inhibition and RNA export. One promising LPA is a miRNA which inhibit virus expression.