Figures & data
Figure 1. DHX32 expression is upregulated in HCC cells and patients with HCC. (A) The expression of DHX32 mRNA in HCC cells was determined by RT-PCR assay. (B) The expression of DHX32 protein in HCC cells was detected by Western blot assay. (C) Kaplan-Meier plot was used to analyze the overall survival of patients with HCC based on DHX32 expression. The medium DHX32 expression (501) was used as a cut-off. **p < .01 and ***p < .001 compared with LO2 cells.
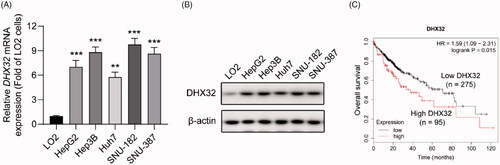
Figure 2. Ectopic expression of DHX32 induces EMT and enhances the migration, invasion, and proliferation of HCC cells. (A) The expression of DHX32 mRNA in Huh7 cells were determined by RT-PCR assay (B) The expression of DHX32, EMT-related proteins (E-cadherin, N-cadherin, and vimentin) were examined by Western blot assay. (C) The mRNA expression of E-cadherin (CDH1), N-cadherin (CDH2), and vimentin (VIM) in Huh7 cells was determined by RT-PCR assay. (D) Cell migration was tested by wound-healing assay and quantification of cell migration is shown. Magnification, 200 ×. (E) Cell invasion was determined by Transwell invasion assay and quantification the number of invaded Huh7 cells. Magnification, 200 ×. (F)Cell proliferation was detected by EdU cell proliferation assay and quantification of the number of EdU-stained cells. Scale bar, 200 μm. Representative images are shown and data are presented as mean ± SEM (n = 5). *p < .05, **p < .01, and ***p < .001 compared with the Vector groups.
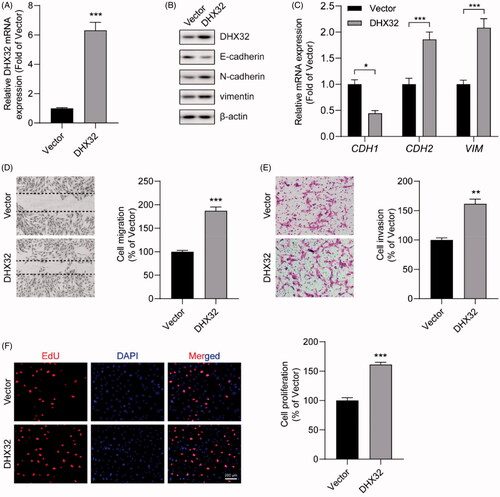
Figure 3. The knockdown of DHX32 reverses EMT and inhibits the migration, invasion, and proliferation of HCC cells. (A) The expression of DHX32 mRNA in Huh7 cells transfected with control shRNA or DHX32 shRNA were examined by RT-PCR assay. (B) The expression of DHX32, E-cadherin, N-cadherin, and vimentin) were determined by Western blot assay. (C) E-cadherin, N-cadherin, and vimentin mRNA expression in Huh7 cells were measured by RT-PCR assay. (D)The effect of DHX32 shRNA on Huh7 cell migration was examined by wound-healing assay. Magnification, 200 ×. (E) The invasive capacity Huh7 cells transfected with DHX32 shRNA was tested by Trans well invasion assay and the number of invasive cells was calculated. Magnification, 200 ×. (F)The proliferation rate of Huh7 cell proliferation after transfection with DHX32 shRNA was determined by EdU cell proliferation assay. The number of EdU-positive cells was quantified. Scale bar, 200 μm. Representative images are shown and data are presented as mean ± SEM (n = 5). **p < .01 and ***p < .001 compared with the Control shRNA groups.
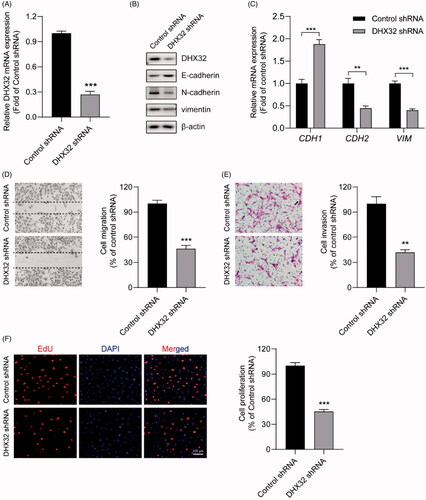
Figure 4. DHX32 regulates the activation of β-catenin pathway in Huh7 cells. (A) The expression of β-catenin in DHX32-overexpressing HCC cells was determined by RT-PCR assay. (B) The protein level of β-catenin in nucleus of Huh7 cells was detected by Western blot assay. (C) The mRNA expression of β-catenin pathway target genes (CCND1, COX2, and MMP7) and WIF1 in DHX32-overexpressing Huh7 cells was examined by RT-PCR assay. (D)RT-PCR assay for the mRNA expression of Huh7 cells with DHX32 knockdown. (E) The effect of DHX32 shRNA on β-catenin expression in nucleus of Huh7 cells was determined by Western blot assay. (F) RT-PCR assay was used to detect the mRNA expression ofCCND1, COX2, MMP7, and WIF1 in Huh7 cells transfected with DHX32 shRNA. Data are presented as mean ± SEM (n = 5). *p < .05, **p < .01, and ***p < .001 compared with the Vector- or Control shRNA-transfected groups.
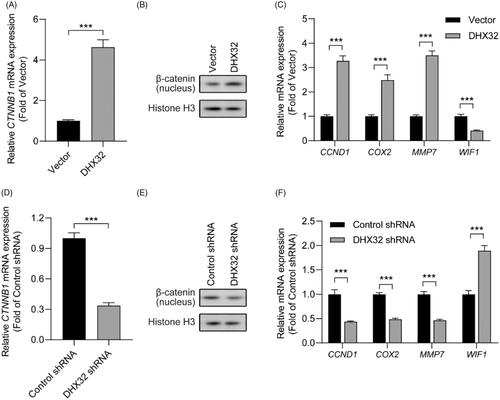
Figure 5. Silencing β-catenin inhibits DHX32-induced HCC progression. (A) RT-PCR assay for the expression of β-catenin (CTNNB1) mRNA in Huh7 cells. (B) RT-PCR assay was used to detect the expression of β-catenin (CTNNB1) mRNA in Huh7 cells after co-transfection with DHX32 and β-catenin siRNA. (C)Representative blot of the expression of β-catenin in nucleus, E-cadherin, N-cadherin, and vimentin in Huh7 cells with DHX32 overexpression plus β-catenin knockdown. (D) RT-PCR assays were performed to determine the expression of E-cadherin, N-cadherin, and vimentin in Huh7 cells. (E) Quantification of the number of EdU-positive Huh7 cells. (F) Wound-healing assay was used to detect the effect of silencing β-catenin on migration in DHX32-overexpressing HCC cells. Representative images and quantification of cell migration are shown. Magnification, 200 ×. (G) Representative images and quantification of the invaded Huh7 cells after co-transfected with DHX32 lentiviral particle and β-catenin siRNA. Data are presented as mean ± SEM (n = 5). *p < .05, **p < .01, and ***p < .001.
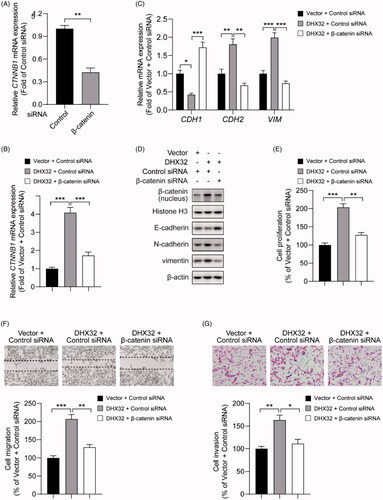
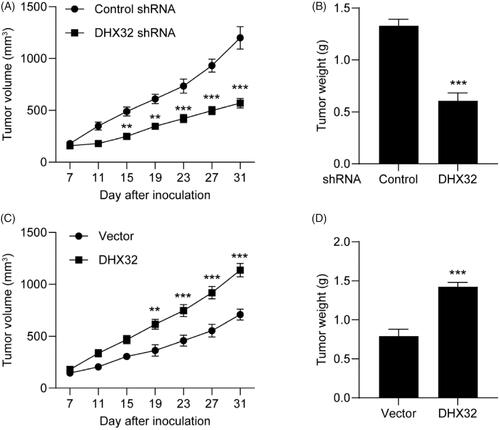
Figure 6. DHX32 inhibition blocks the growth of HCC xenograft tumours. Huh7 cells were stably transfected with DHX32 lentiviral particle or DHX32 shRNA, and inoculated into the flank of male BALB/c Nude mice. Tumour volume and tumour weight were analyzed. (A-B) The volume change (A) and weight (B) of tumours in mice injected with Huh7 cells stably transfected with DHX32 shRNA or control shRNA. (C–D) The volume change (C) and weight (D) of tumours in mice injected with Huh7 cells stably overexpressed DHX32 or vector. Data are presented as mean ± SEM (n = 6). **p < .01 and ***p < .001 compared with tumours in mice injected with Huh7 cells stably transfected with control shRNA or vector.
Availability of data and materials
All data and materials relevant to this study are available in the article.
