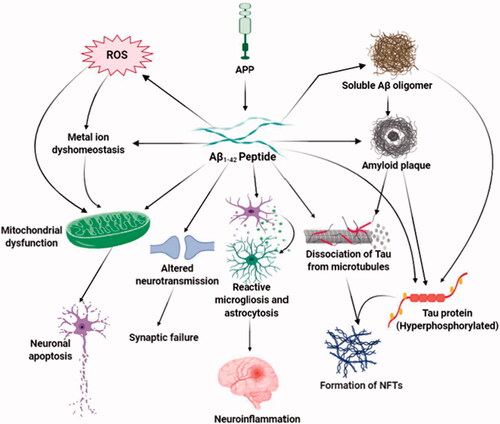
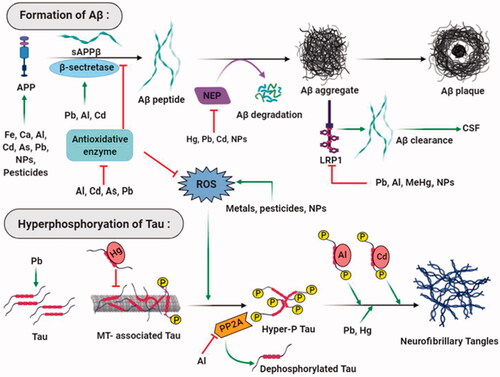
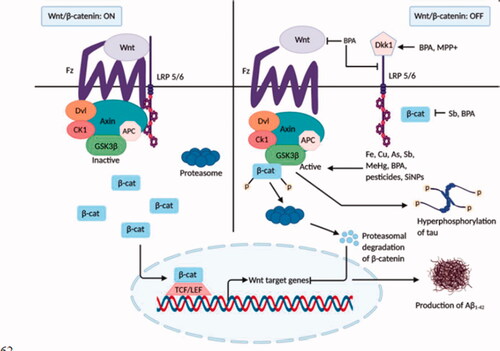
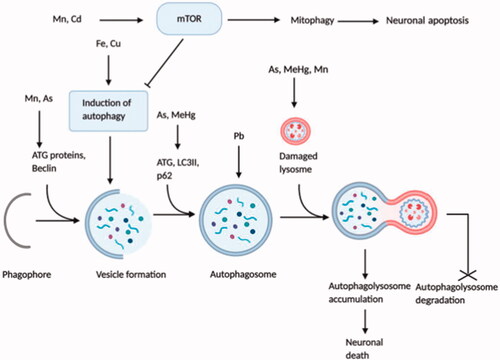
Figures & data
Table 1. Signalling pathways modulated by neurotoxicants in the pathogenesis of Alzheimer’s disease.
Table 2. Role of neurotoxin-induced cellular stresses in the progression of Alzheimer’s disease.
Table 3. Effects of neurotoxic agents on iGluRs and mGluRs in the onset and progression of Alzheimer’s disease.