Figures & data
Table 1. Characteristics of the PSAD non-initiators and initiators and standardized mean differences of variables in the original non-matched and matched sample.
Figure 1. Estimated cumulative incidence curves in competing risks analyses for a) non-fatal vascular event vs. vascular and non-vascular mortality occurring before vascular event and b) non-fatal restroke vs. restroke and non-restroke mortality occurring before restroke in patients initiating post-stroke antidepressants (PSAD) within one year from ischaemic stroke. Eight PSAD initiators and 16 matched controls were excluded from the vascular event dataset (n = 585), and 2 initiators and 4 matched controls were excluded from the recurrent stroke dataset (n = 603), since in these cases the PSAD use started after the event of interest.
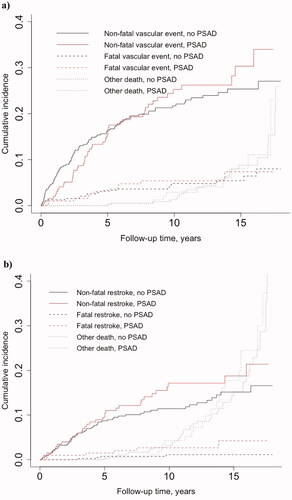
Table 2. Association between initiation of post-stroke antidepressants and mortality, any vascular event and recurrent stroke.
Supplemental Material
Download PDF (142.2 KB)Data availability statement
We have documented the data, methods, and materials used to conduct the research in this report. The individual patient data are not publicly available due to legal restrictions. The syntax code for study analyses in R software [Citation26] is available from the corresponding author, J.B., upon reasonable request.
