Figures & data
Table 1. Study inclusion and exclusion criteria.
Figure 1. Study visit overview. All four study visits will consist of four elements: (1) assessment of inclusion criteria related to provoked vulvovaginal pain during the tampon test or by self-report of insertional sexual activity and/or with touch to the vaginal opening, (2) completion of validated questionnaires to measure perceived pain, health, mood, and sexual function, (3) quantitative sensory testing to measure pressure pain thresholds at local and remote body sites, and (4) collection of biologic samples to measure local (vaginal) and systemic (blood) cytokines and miRNAs.
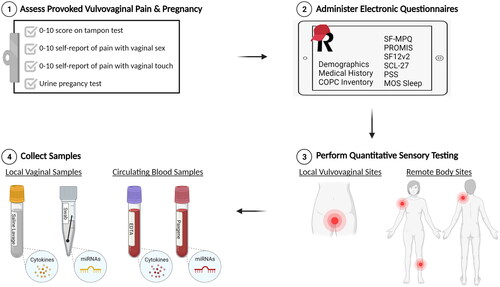
Table 2. Study procedures and assessments in chronological order.
Supplemental Material
Download PDF (538.6 KB)Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
