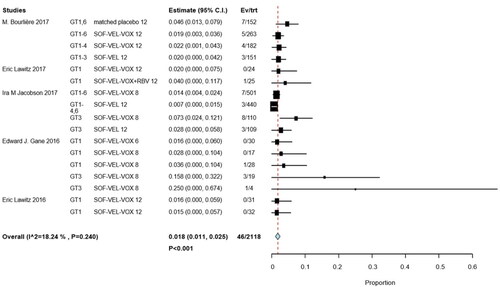
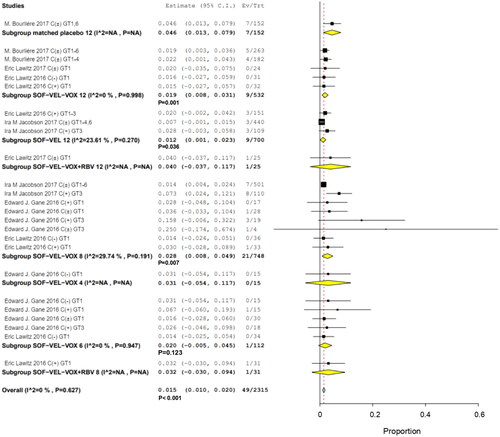
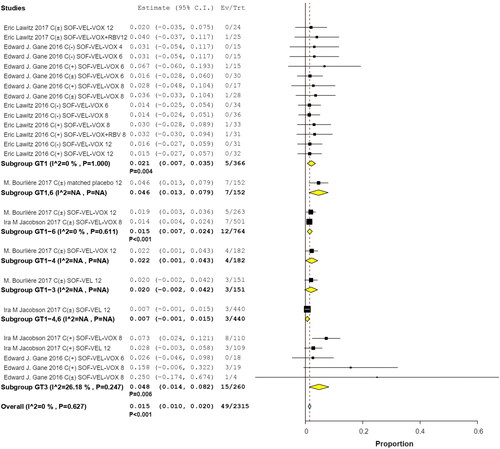
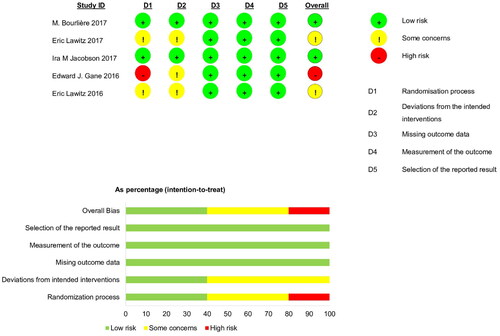
Figures & data
Table 1. Summary and baseline data of patients in included studies.
Table 2. Others grade 3 laboratory abnormality.
Data availability statement
No data is available for this study.