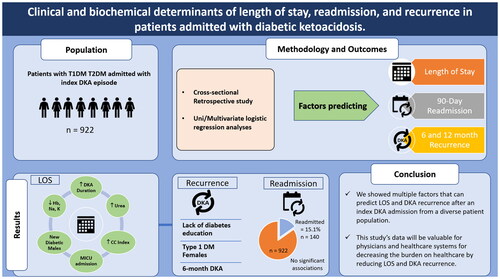
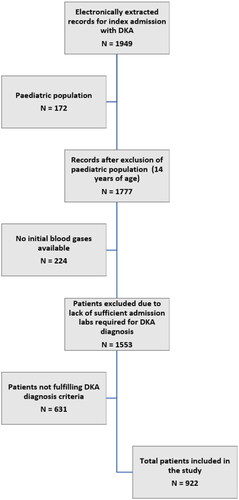
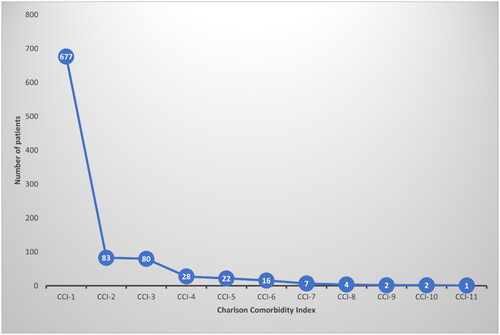
Figures & data
Table 1. Characteristics of the patients admitted with index DKA episode.
Figure 3. Frequency of diabetes related medication use among the study cohort. (3a) Use of insulin types. (3b) Use of oral anti-diabetic medicines.
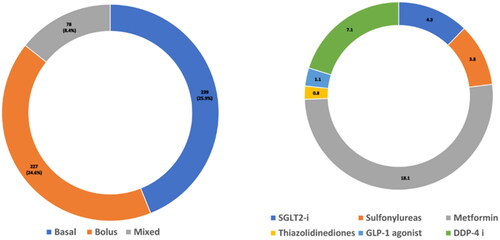
Figure 4. Reported triggering factors of DKA development during the index admission in the study cohort (iatrogenic: inadequate medication dosage prescribed, medications side-effects: DKA secondary to SGLT2 inhibitors, DDP-4 inhibitors and GLP-1 agonists).
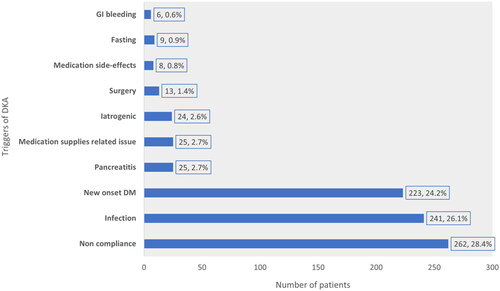
Table 2. Clinical outcomes of patients admitted with index DKA episodes (N = 922).
Table 3. Multivariate linear regression analysis model for the factors predicting length of stay.
Table 4. Multivariate logistic regression analysis model with predictors of 12-month recurrence. Corrected for age, length of stay and ethnicity (N = 922).
Supplemental Material
Download MS Word (43.2 KB)Supplemental Material
Download PDF (124.9 KB)Data availability statement
Available from the corresponding author upon reasonable request.