Figures & data
Figure 1. Characteristics of endothelial-to-mesenchymal transition. Morphology changes from an oval shape to a long spindle shape. Gene expression alterations include the loss of endothelial protein marker expression such as von Willebrand factor (vWF), platelet-endothelial cell adhesion molecule-1 (PECAM-1; also known as CD31), vascular-endothelial cadherin (VE-cad), vascular endothelial growth factor receptor 2 (VEGFR2), Tie2 and ZO-1 and the acquisition of mesenchymal marker expression such as α-smooth muscle actin (α-SMA), fibroblast activation protein (FAP), vimentin, fibronectin, N-cadherin (N-cad), fibroblast specific protein 1 (FSP1) and collagen type I. Functional conversion is related to the loss of intercellular junctions and angiogenic capacity, an increase in vascular permeability and the acquisition of migratory and invasive properties. Adapted from ‘Tumor Microenvironment with Callout (Layout)’, by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates.
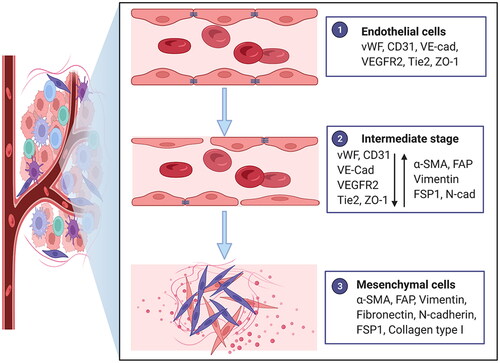
Table 1. The common terms and abbreviations of EndoMT.
Table 2. EndoMT in human tumour tissue specimens.
Figure 2. Factors and molecular mechanisms that regulate endothelial-to-mesenchymal transition (EndoMT) in tumour progression. Tumour cells induce EndoMT: Tumour cells activate the Notch pathway via Jag1 and DLL1 to induce EndoMT. Tumour cells secrete transforming growth factor-beta (TGF-β), hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF) and α1-antitrypsin (A1AT) to induce EndoMT. TGF-β enhances the phosphorylation of Smad2/3 and the upregulation and phosphorylation of tubulin-β3 to induce EndoMT, and interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α) and Notch may play synergistic roles. HGF induces the phosphorylation of c-Met, and the expression of ETS-1 enhances the expression of matrix metalloproteinase-14 (MMP-14), which mediates the degradation of vascular-endothelial cadherin (VE-cad). PDGF binds to PDGFR and induces its phosphorylation, which activates NF-κB. Activation of NF-κB upregulates the expression of Snail, thus inhibiting the transcription of vascular endothelial growth factor receptor 2 (VEGFR2). Tumour cells release extracellular vesicles enriched with TGF-β (eTGF-β) and miR-92a to induce EndoMT. The tumour microenvironment (TME) induces EndoMT: Cancer-associated fibroblasts (CAFs) secrete interleukin-6 (IL-6), TGF-β1 and TGF-β2 to induce EndoMT. Tumour-associated macrophages (TAMs) induce EndoMT through the secretion of osteopontin (OPN). OPN-integrin αvβ3 engagement activates the PI3K/Akt pathway and hypoxia-inducible factor-1α (HIF-1α) expression, which in turn transactivates TCF12 gene expression. TCF12 transcriptionally represses the VE-cad gene. Interstitial fluid flow (IFF) and extracellular matrix (ECM) upregulate EndoMT. Systematic factors induce EndoMT: Hypercholesterolemia increases 27-Hydroxycholesterol (27HC) expression and induces EndoMT via the acetylation and phosphorylation of STAT3. Therapeutic interventions induce EndoMT: Radiotherapy activates the Notch signalling pathway and upregulates p53 to induce EndoMT. Chemotherapy can also induce EndoMT. Adapted from ‘The Hippo Tumor-suppressor Pathway’, by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates
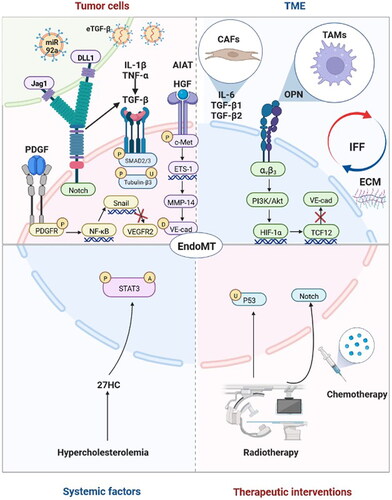
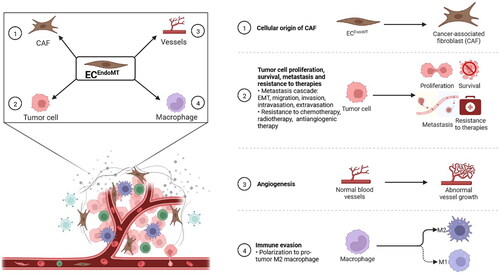
Figure 3. Endothelial-to-mesenchymal transition (EndoMT) promotes tumour progression. ① Endothelial cell undergoing EndoMT (ECEndoMT) mediates the cellular origin of cancer-associated fibroblast (CAF). ② ECEndoMT promotes tumour cell proliferation, survival and metastasis involving epithelial-mesenchymal transition (EMT), migration, invasion, intravasation and extravasation and resistance to chemotherapy, radiotherapy and antiangiogenic therapy. ③ ECEndoMT induces angiogenesis. ④ ECEndoMT enhances immune evasion by inducing M2 polarization of macrophage. Adapted from ‘The Tumor Microenvironment: Overview of Cancer-Associated Changes’, by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study
