Figures & data
Figure 1. Questionnaire algorithm used by the call center during the telephone survey following the first dose TURKOVAC vaccine, Turkey, 10–17 January 2022. The ‘Other’ adverse events stated in the algorithm were open-ended. During the call, the ‘other’ adverse events reported by the participants were noted as open-ended text.
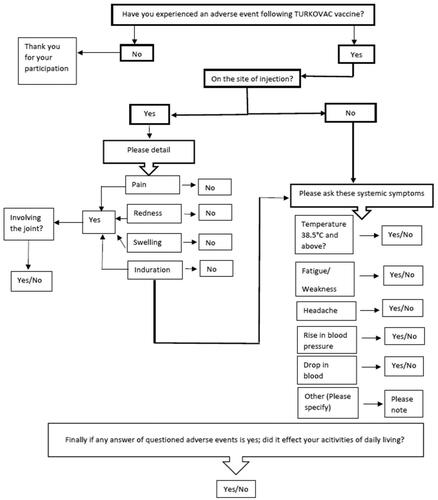
Table 1. Baseline characteristics of the participants by follow-up day.
Table 2. Sex and age distribution of the participants by follow-up day.
Figure 2. Distribution of participants by the number of doses of total COVID-19 vaccines (including the TURKOVAC vaccine as the latest dose) by follow-up days.
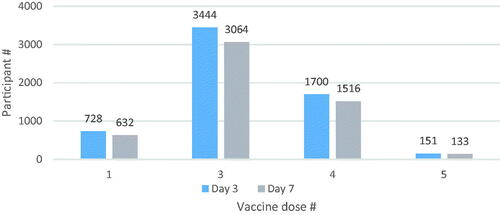
Table 3. Rates of adverse events reported by the participants by follow-up day.
Table 4. Comparative analysis of adverse events among participants who received TURKOVAC as their first COVID-19 vaccine compared to participants who received TURKOVAC as a booster dose.
Table 5. Adjusted odds for selected adverse events following the first dose of TURKOVAC vaccine.
Data availability statement
Raw data were generated at SABIM. Derived data supporting the findings of this study are available on request from the corresponding author, A.K. The data are not publicly available as the information contained may compromise the privacy of research participants.
