Figures & data
Table 1. Case reports and studies of BP patients treated with dupilumab.
Figure 1. IL-4 and IL-13 receptor structure IL-4 can bind to both type I and type II receptors. (A) Type I receptors consist of IL-4Rα and γc, whereas type II receptors consist of IL-4Rα and IL-13Rα1. When IL-4 binds to a type I receptor, Janus kinase (JAK) 1 and JAK3 are activated; both can induce tyrosine phosphorylation of the type I receptor intracellular domain, forming docking sites for downstream signaling molecules, such as signal transducer and activator of transcription (STAT) 6 and insulin receptor substrate-2 (IRS-2). Homodimers of STAT6 then translocate to the nucleus to facilitate transcription of IL-4- and IL-13-dependent genes. IRS-2 can activate signaling molecules, such as PI3K to induce gene transcription. (B) IL-13 binds to IL-13Rα2 with greater affinity than IL-13Rα1; the IL-13Rα2 receptor is considered a decoy receptor because it lacks a cytoplasmic signaling tail. However, the cytoplasmic domain of IL-13Rα2 may attenuate IL-4 signaling by inhibiting dimerization with γc or IL-13Rα1. (C) When IL-4 or IL-13 binds to a type II receptor, JAK1 and JAK2/TYK2 are activated.
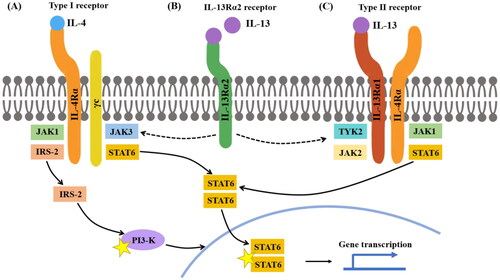
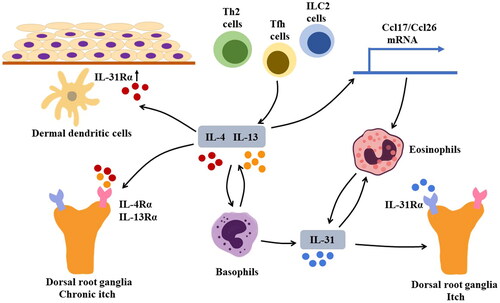
Figure 2. Mechanism of pruritus in BP Sources of IL-4 and IL-13 may include T helper 2 (Th2) cells, group 2 innate lymphoid cells (ILC2s), and follicular helper T (Tfh) cells. IL-4 and IL-13 can activate basophils that produce IL-31, a cytokine that induces pruritus by stimulating IL-31Rα on sensory neurons. Additionally, basophils can produce IL-4 and IL-13; IL-31 can recruit eosinophils to lesion sites, contributing to the formation of a positive feedback loop that exacerbates pruritis. In addition to IL-31, IL-4, and IL-13 can directly cause chronic pruritus by interacting with IL-4Rα and IL-13Rα on sensory neurons. Furthermore, IL-4 can induce upregulation of IL-31Rα on dermal dendritic cells. The augmented IL-31/IL-31Rα interaction can lead to increased production of CCL17 and CCL22, thus promoting Th2-related immune response.
Supplemental Material
Download MS Word (17.5 KB)Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
