Figures & data
Figure 1. Study design (the phase discussed in this manuscript is in the shaded box).
T0, study entry; Y, year prior to study entry.
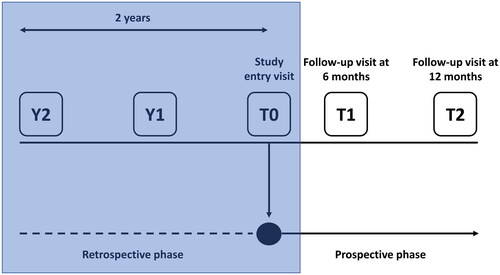
Table 1. Patient demographic and clinical characteristics.
Figure 2. Non-motor and motor symptoms of Parkinson’s disease (assessed by parts I/II of MDS-UPDRS). (A) part IA (physician assessed) nM-EDL; (B) part IB (patient assessed) nM-EDL; and (C) part II (patient assessed) motor experiences of daily living.
MDS-UPDRS: Unified Parkinson’s Disease Rating Scale by the Movement Disorder Society; nM-EDL: non-motor experiences of daily living.
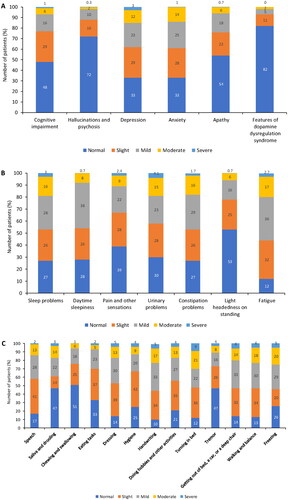
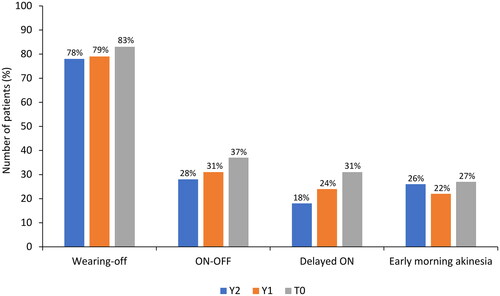
Figure 4. Improvements in motor and non-motor symptoms of wearing-off fluctuations (as assessed by the WOQ-19).
WOQ-19: 19-item Wearing-Off Questionnaire.
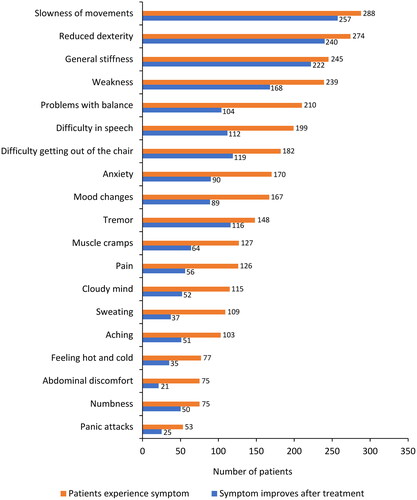
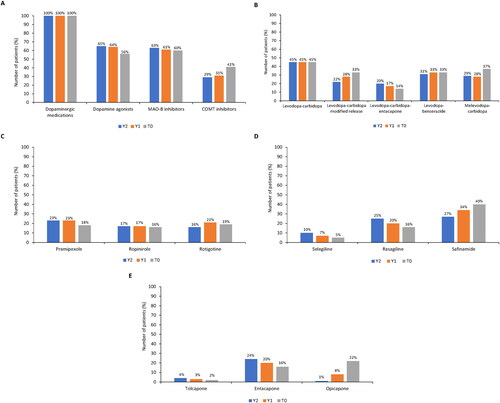
Figure 5. Parkinson’s disease treatments 2 years prior (Y2 and Y1) to study entry (T0). (A) pharmacologic treatment (levodopa-carbidopa-entacapone was included in two classes - dopaminergic medications and COMT inhibitors); (B) dopaminergic medications (including concomitant use of >1 medication or combination); (C) dopamine agonists; (D) MAO-B inhibitors; and (E) COMT inhibitors (entacapone included levodopa-carbidopa-entacapone).
COMT: catechol-O-methyltransferase enzyme; MAO-B: monoamine oxidase B enzyme.
Supplemental Material
Download MS Word (44.3 KB)Data availability statement
The data supporting the findings of this study are available from the study sponsor (Bial Italy) upon reasonable request ([email protected]).