Figures & data
Table 1. List of inclusion and exclusion criteria.
Figure 1. Visual representation of study design and follow-up visits. HAM-D17: Hamilton Depression Rating Scale 17 item version; MDI: Major Depression Inventory; WHO-5 World Health Organization Quality of Life Index; PSQI: Pittsburg Sleep Quality Index; SIDAS: Suicidal Ideation Assessment Scale; YMRS: Young Mania Rating Scale; UKU: The UKU Side Effect Rating Scale; SCIP: The Screen for Cognitive Impairment in Psychiatry. TMT-B: Trail Making Test Part B; FERT-Facial Emotion Recognition Task; ECMT: Emotional Categorization and Memory Test; SSVEPs: Steady State Visually Evoked Potentials.
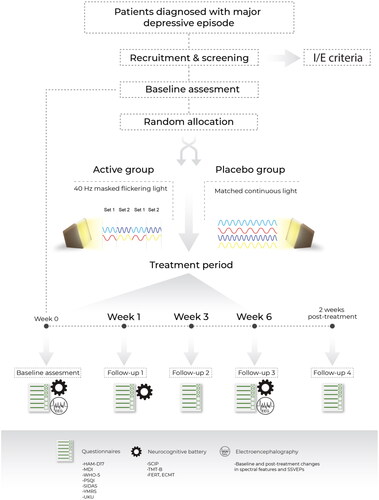
Figure 2. Representation of light paradigms and the design of invisible spectral flicker. Set 1: Cyan and red, set 2: blue and yellow.
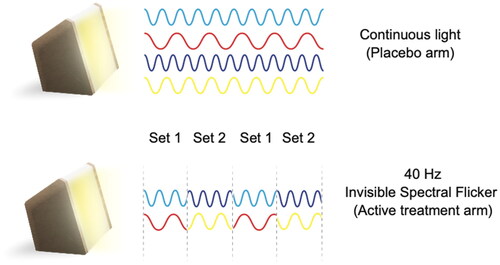
Table 2. Light matching in two different treatment conditions.
Table 3. Summary of study endpoints.
Data availability statement
After publication of the results, anonymized data can be made available from the principal investigator (K.M.) upon reasonable request.
