Figures & data
Figure 1 Proposed alternative sources of stem cells for a cell‐based therapy in Parkinson's disease. These sources include pluripotent embryonic stem cells and multipotent region specific stem cells that can be isolated from embryonic tissues, such as midbrain, and from the adult, such as subventricular zone and bone marrow.
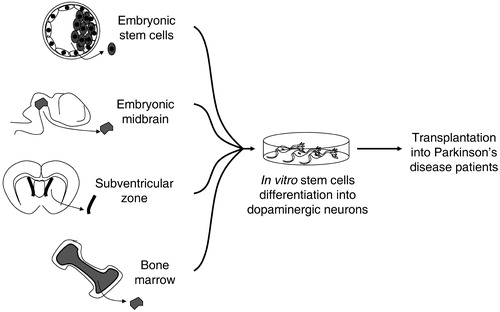
Figure 2 General basic steps of the methods developed so far forin vitro differentiation of hESCs into dopaminergic neurons. EBs, embryoid bodies; EGF, epidermal growth factor; bFGF, basic fibroblast growth factor; FGF‐8, fibroblast growth factor‐8; SHH, sonic hedgehog.
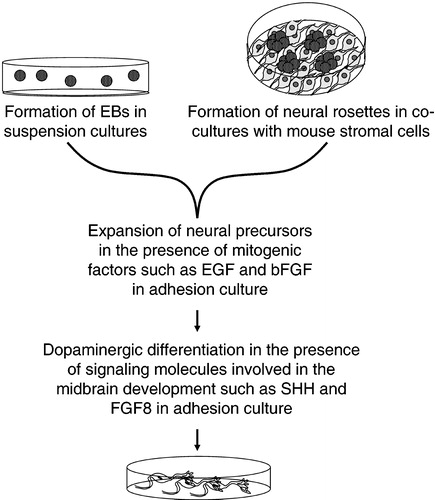
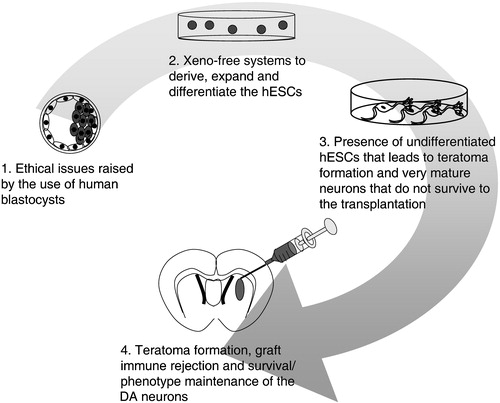
Figure 3 Present limitations in the development of the hESC‐based therapy for PD. Limitations are found: 1) in the derivation of new hESCs lines, 2) in the culture of these cells, 3), in the derivation of the appropriate cells for successful and safe transplantation, and 4) in the survival and integration of the grafted cells.