Figures & data
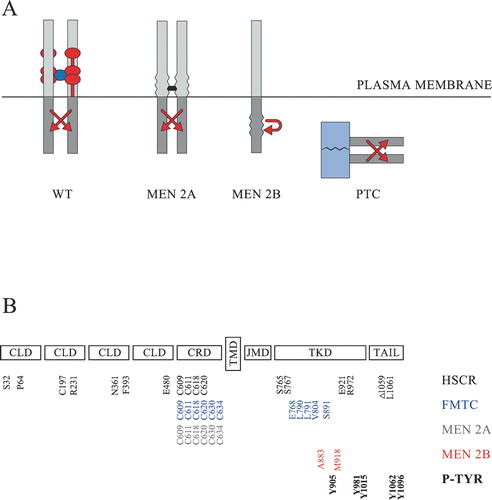
Figure 1 Schematic representation of RET and its oncogenic variants. A: Normally wild‐type RET (WT) is activated at the cell surface in the presence of a tetrameric GFL/GFRα complex. The dimeric GFL is shown in dark blue. The GPI‐anchored GFRα co‐receptors are normally comprised of three cysteine‐rich domains and are here shown in red. The most common multiple endocrine neoplasia type 2A (MEN 2A) variants are activated through the formation of abnormal covalent S‐S bridges (shown in black) between the extracellular domains of two RET molecules. The MEN 2B variants harbour activating mutations in the intracellular kinase domain and may activate signalling cascades either as monomers or as non‐covalently associated dimers. The papillary thyroid carcinoma (PTC) variants are not membrane‐bound, and the activation of their tyrosine kinase domains (shown in grey) is driven by the dimerizing nature of an N‐terminally fused protein (shown in light blue). B: The extracellular part of RET consists of four cadherin‐like domains (CLD) and a cysteine‐rich domain (CRD). The intracellular part consists of a juxtamembrane domain (JMD), a tyrosine kinase domain (TKD) and a C‐terminally located tail. The transmembrane domain (TMD) is located in the mid‐part of the protein. Part of the mutated amino acids which have been shown to be associated with Hirschsprung's (HSCR) disease (S32, P64, C197, R321, N361, F393, E480, C609, C611, C618, C620, S765, S767, E921, R972, Δ1059, L1061 in black), familial medullary thyroid carcinoma (FMTC) (C609, C611, C618, C620, C630, C634, E768, L790, L791, V804, S891 in blue), MEN 2A (C609, C611, C618, C620, C630, C634 in grey), or MEN 2B (A883, M918 in red) are shown at the bottom, together with the main phosphorylated tyrosines (P‐TYR) (Y905, Y981, Y1015, Y1062, Y1096 in bold) having an impact on normal RET‐signalling.