Figures & data
Figure 1. Iron uptake pathway in mammalian cells. The majority of iron is taken up by mammalian cells via transferrin (Tf) which receives ferric iron from macrophages or enterocytes. 1) Holo-Tf, after binding to its cognate receptor, transferrin receptor (TfR) on the cell surface, enters the cell via receptor-mediated endocytosis. This process begins with the formation of a clathrin-coated pit, which matures into an early endosome. 2) The early endosome further matures, and protons enter by the action of the V-type ATPase. 3) When the pH of the endosomes becomes more acidic than 5.5, the Fe3+ is released from the Tf-TfR complex. Iron must then be reduced, probably by a member of the Steap family of proteins, before its translocation out of the endosome by DMT1. The next steps of iron distribution within the cell are less well understood. 4) Either iron is handed off from DMT1 directly to mitochondria from the endosome, utilizing mitoferrin (Mfn) to cross the inner membrane, and the excess portion may subsequently be re-exported to cytosolic ferritin (Ft). 5) Alternatively or additionally, iron leaves endosomes to enter a labile iron pool within the cytosol (LIP), from where it may then be translocated into the mitochondria via Mfn or become loaded into Ft. Once in the mitochondrial matrix, the metal is utilized by either ferrochelatase (FC) to generate heme or the mitochondrial iron-sulfur cluster (ISC) assembly machinery. 6) Apo-Tf (which remains bound to the TfR under acidic conditions) and the TfR recycle to the cell surface, where apo-Tf is released into the plasma to become reloaded with Fe3+.
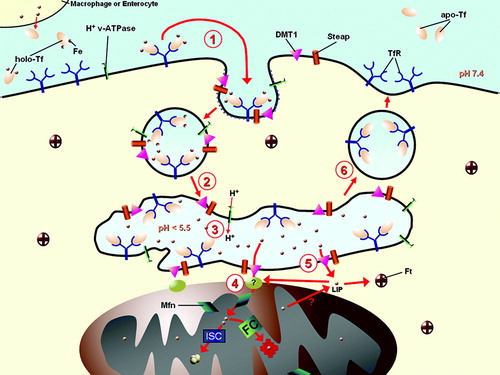
Figure 2. Working model of iron-sulfur cluster biogenesis. In the mitochondria, the complex of Nfs1 and Isd11 functions as a cysteine desulfurase to generate the sulfur needed for biogenesis. An Fe/S cluster is then assembled on the Isu1 scaffold, with ferrous iron transported into the organelle by Mrs3/4 and possibly delivered by Yfh1. Presumably, the electron transfer chain from NADH to the ferredoxin Yah1 serves to reduce the sulfur atom to sulfide. A chaperone system, which includes the Hsp70-like protein Ssq1, the J-type co-chaperone Jac1, and the nucleotide exchange factor Mge1, transfers de novo assembled clusters from the scaffold to apo-proteins. Grx5 also participates in some capacity in this cluster delivery system. Isa1, Isa2, and Iba57 all have a specific role in the maturation of a subset of apo-proteins, which includes aconitase-type proteins and radical-SAM enzymes. The ISC export machinery, comprised of the ABC transporter, Atm1, the intermembrane space sulfhydryl oxidase, Erv1, and glutathione (GSH) moves an intermediate (X) produced by the mitochondrial ISC system to the cytosol. This intermediate facilitates Fe/S protein maturation in the cytosol and nucleus, which further requires the cytosolic Fe/S protein assembly (CIA) machinery. In this pathway, a transient Fe/S cluster is initially formed on the complex of the P-loop NTPases, Cfd1 and Nbp35, which thus serve as scaffolds for Fe/S cluster assembly. Delivery of the Fe/S clusters from the scaffold complex to apo-proteins requires the hydrogenase-like protein Nar1 and the WD40 repeat protein Cia1.
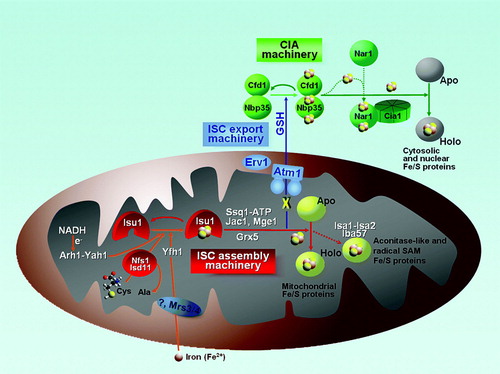
Figure 3. Iron homeostasis is regulated by Fe/S protein assembly systems. (A): In yeast, the ISC assembly machinery produces an unknown substrate which is exported to the cytosol by Atm1. The substrate, which may be identical to the substance needed for Fe/S cluster formation on Cfd1/Nbp35 (see ), prevents the translocation of the transcription factors Aft1/2 into the nucleus and the activation of the iron regulon. Thus, insufficiency in any part of the mitochondrial ISC machineries results in a ‘low iron’ signal which is mediated by Aft1/Aft2 and possibly other proteins including Grx3/Grx4 and Fra1/Fra2 to result in the activation of the iron regulon. (B): In mammalian tissues, the presence or absence of an Fe/S cluster on IRP1 serves as a binary switch, determining the binding capacity of the protein to mRNA stem loop structures serving as iron-responsive elements (IRE). When the Fe/S cluster is absent, IRP1 has the ability to bind to IREs, thereby stabilizing mRNAs with 3’-UTR IREs (e.g. transferrin receptor 1) and blocking translation of mRNAs with 5’-UTR IREs (e.g. ferritin). Hence, appropriate regulation of iron handling by mammalian cells requires not only the mitochondrial ISC machineries including the substrate exported by ABCB7, but also a functional cytosolic assembly system (CIA).
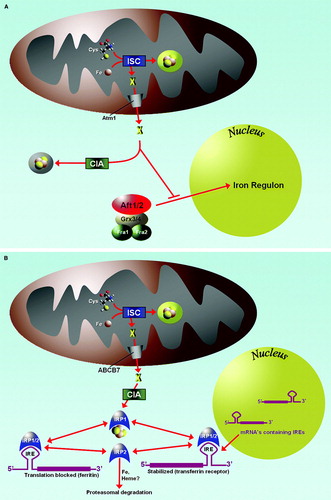
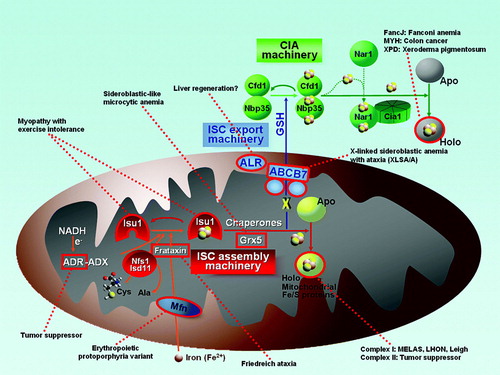
Figure 4. Diseases resulting from deficient Fe/S proteins. Fe/S protein biogenesis components or Fe/S proteins that are associated with human diseases are outlined in red. (ADR = adrenodoxin reductase; ADX = adrenodoxin; Mfn = mitoferrin; ALR = augmenter of liver regeneration (Erv1 homologue); MELAS = mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes; LHON = Leber hereditary optic neuropathy).