Figures & data
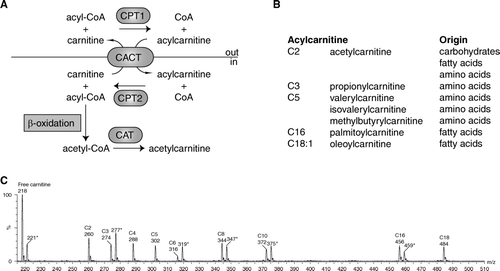
Figure 1. A: Schematic representation of acylcarnitine metabolism. Fatty acids are activated in the cytosol to acyl-coenzyme A (CoA). Carnitine palmitoyl transferase 1 (CPT1) converts the acyl-CoA into an acylcarnitine, which can subsequently be transported across the mitochondrial inner membrane by the carnitine acylcarnitine carrier (CACT, SLC25A20). CPT2 converts the acylcarnitine back into an acyl-CoA, which can subsequently be β-oxidized. Carnitine acetyltransferase (CAT) catalyzes the reversible transfer of short chain acyl groups, such as acetyl and propionyl, between CoA and carnitine. B: Selected acylcarnitines and their origin. C: An acylcarnitine spectrum. Acylcarnitines are often measured with tandem mass spectrometry (MSn) using a so-called parent ion scan. In this approach, molecules are fragmented in a collision cell. After fragmentation only those molecules are determined that give a common, often specific, fragment ion. The acylcarnitine spectrum is derived by scanning the first mass spectrometer, followed by fragmentation in a collision cell and only allowing a specific acylcarnitine fragment ion of mass-to-change ratio (m/z) 85 to pass through the second mass spectrometer to be detected. Selected acylcarnitines are indicated in the spectrum. The m/z value corresponds to the protonated butyl ester of the acylcarnitine. The m/z values marked with an asterisk (*) are the internal standards for free carnitine (221), C3-carnitine (277), C6-carnitine (319), C8-carnitine (347), C10-carnitine (372), and C16-carnitine (459). Each internal standard has an m/z value +3, because it is labeled with three deuterium atoms (2H).