Figures & data
Table I. Cell types expressing RAGE (partial list): examples of the impact of RAGE ligands and selected references.
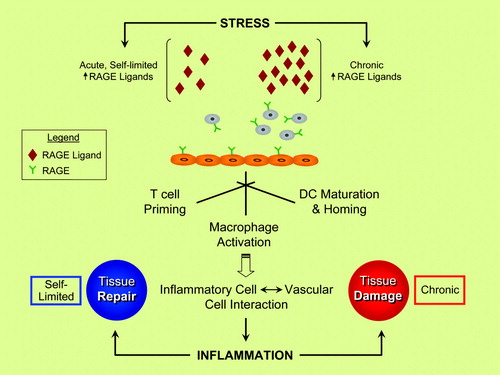
Figure 1. RAGE and inflammatory responses—repair versus injury? Evidence suggests that the ligand families of RAGE (receptor for advanced glycation end-products) are generated by both acute and chronic stresses. RAGE is expressed by key cell types linked to inflammatory responses, such as neutrophils, T lymphocytes, monocytes/macrophages, and dendritic cells (DC). Activation of RAGE in these cell types stimulates inflammation mechanisms, and, via RAGE ligand-stimulated upregulation of adhesion molecules and chemokines on endothelial cells (ECs), sets the stage for vascular inflammation and perturbation—harbingers of tissue damage. In acute stress, such as in injury to the peripheral nervous system, rapid and self-limited upregulation and release of RAGE ligands stimulate inflammatory mechanisms that contribute to tissue repair. In marked contrast, in chronic disease settings such as diabetes, autoimmunity, aging, and neurodegeneration, the long-term and sustained accumulation of RAGE ligands in the tissues crosses a threshold at some level, thereby triggering mechanisms that mediate chronic stress and, ultimately, tissue damage. A key challenge in the translational biology of RAGE is the identification of strategies to block the maladaptive consequences of RAGE ligation while preserving innate adaptive repair pathways.