Figures & data
Table I. Characteristics of the selected population involved in spontaneous preterm birth.
Table II. Allele and genotype frequencies of the SFTPC SNP rs4715 in preterm (gestational age <36 wk) and term (gestational age ≥ 37 wk) infants, in mothers with preterm deliveries and in mothers with exclusively term deliveries.
Figure 1. SP-C expression in mouse gestational tissues is not affected by LPS. A–E: The changes in the mRNA levels of SP-C in mouse tissues as a response to maternal LPS were analyzed by RPA in maternal lung (A), fetal lung (B), uterus (C), fetal membranes (D), and placenta (E) and normalized to the expression level of the housekeeping gene L32. The mRNAs levels are shown as fold increases compared to PBS controls. Representative gel electrophoresis of SP-C and L32 is shown for each tissue. Tissues were collected at 17 dpc 3 or 8 hours after PBS or LPS injection for RNA isolation. Analyses of 3–7 dams or their litters are shown as means±SEM. F: RT-PCR (25 cycles) was performed to confirm the SP-C expression in mouse gestational tissues harvested from PBS-treated animals. LPS: lipopolysaccharide, RPA: ribonuclease protection assay, RT-PCR: reverse transcriptase polymerase chain reaction, SP-C: surfactant protein C, PBS: phosphate-buffered saline.
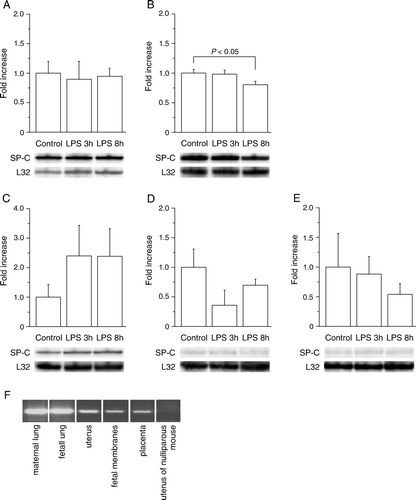
Figure 2. SP-C transcript is similar in mouse lung and gestational tissues. A: For Northern blot the mouse tissues were harvested from PBS-treated mice at 17 dpc. The amount of total RNA used was 10 µg for mouse lung and 30 µg for other mouse tissues. B: In schematic representation of mouse SP-C cDNA the exons are indicated as numbered boxes, and start and stop codons are shown. The locations of primers (F1, F2, F3, R1, and R2) used in the validation of mouse extrapulmonary SP-C cDNA and lengths of the PCR products of SP-C cDNA are indicated. The PCR products were electrophoresed and further verified by sequencing. PBS: phosphate-buffered saline, SP-C: surfactant protein C, PCR: polymerase chain reaction.
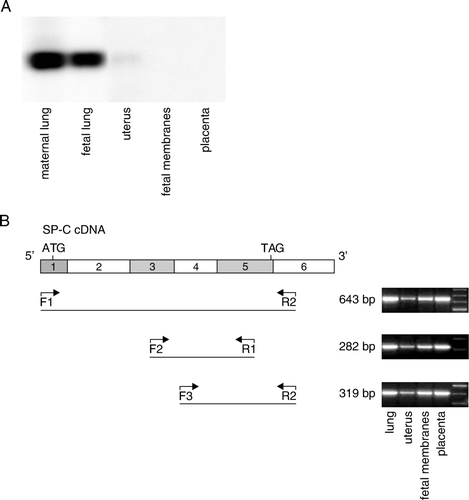
Figure 3. SP-C proprotein is present in mouse gestational tissues. A: In Western analysis different amounts of protein from PBS-treated mice were used (maternal lung 10 µg, fetal lung 20 µg, and uterus, fetal membranes, and placenta 50 µg collected at 17 dpc). The proteins were detected using proSP-C antibody FL-197. B–F: Immunohistological localization of proSP-C in fetal membranes (B–E) and maternal lung at 17 dpc (F); tissue sections from either PBS treated wild-type (B, C, and F) or Sftpc −/− (D, E) mouse stained with proSP-C antibody (WRAB-SP-C). Original magnifications in panels B, D, and F are×20 and in panels C and E×40. Positive staining in epithelial yolk sac cells (B and C) and in alveolar type II cells (F) is indicated by arrows. SP-C: surfactant protein C, PBS: phosphate-buffered saline, SFTPC, SP-C gene.
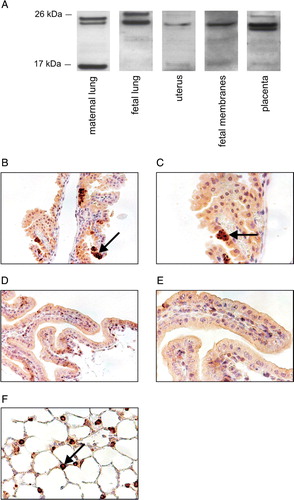
Figure 4. SP-C is expressed in human placenta and fetal membranes. A: The amount of total RNA used in Northern blot: 5 µg for lung and 15 µg for other tissues. B: RT-PCR (30 cycles) was performed to study the SP-C expression in human tissues. Tissues were obtained from normal term delivery or during surgical procedure. RT-PCR: reverse transcriptase polymerase chain reaction, SP-C surfactant protein C gene.
Figure 5. Fetal SFTPC SNP rs4715 associates with the duration of PPROM. Frequencies of the SFTPC rs4715 minor allele and genotypes in (A) fetuses born prematurely with (n=131) or without PPROM (n=176), and in (C) mothers with (n=139) or without PPROM (n=162). The fetuses (B) and mothers (D) with PPROM were divided into two groups based on the interval between of PPROM and delivery: short PPROM (duration <72 hours: 51 fetuses, 52 mothers) and prolonged PPROM (duration ≥ 72 hours; 80 fetuses and 87 mothers). SFTPC: surfactant protein C gene, SNP: single nucleotide polymorphism, PPROM: preterm premature rupture of fetal membranes.
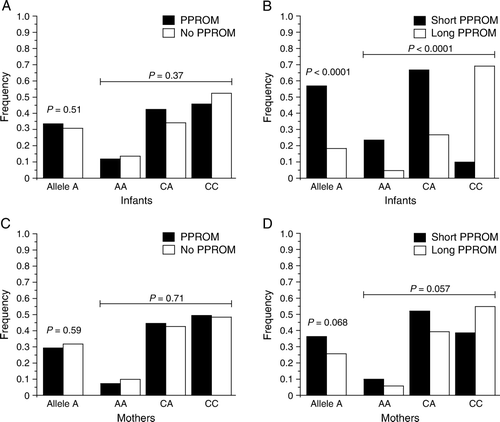
Table III. Duration of PPROM in days according to the fetal or maternal SFTPC rs4715 genotype in 137 mother–fetus pairs.
Supplemtary Table I. Relationship of management practice on the duration of PPROM; analysis according to fetal SFTPC genotype.