Figures & data
Figure 1. Study design. *RAS inhibitors (ACE inhibitors, ARBs, direct renin inhibitors), CCBs, thiazide diuretics, and diuretics similar to thiazides.
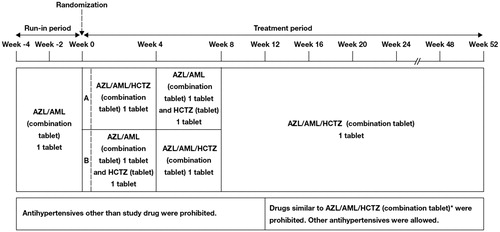
Table 1. Baseline characteristics of all randomized patients.
Table 2. Summary of overall safety data.
Table 3. All-cause AEs occurring in >2% of patients.
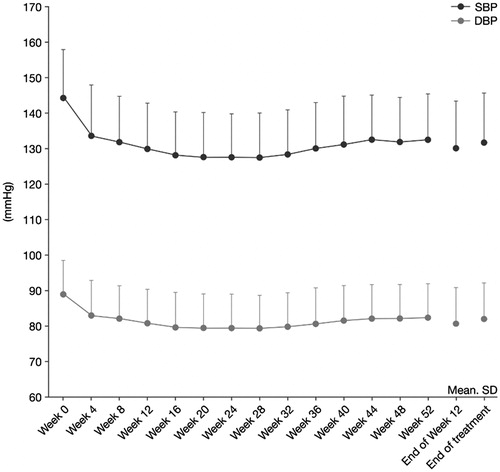
Figure 3. Time profile of mean office trough sitting SBP and DBP. p < .0001 versus week 0 at all post-baseline timepoints.
Table 4. Changes in office trough sitting SBP and DBP and morning home sitting SBP and DBP from baseline at each time point for the FAS.