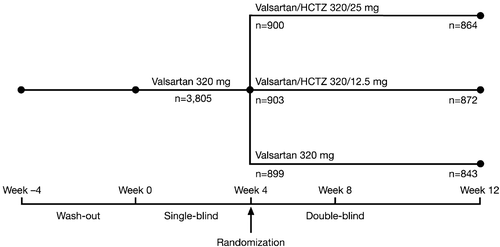
Figures & data
Table I. Patient demographics and characteristics at baseline (randomized population).
Figure 2. Mean sitting diastolic blood pressure(MSDBP) (a) and mean sitting systolic blood pressure (MSSBP) (b) changes from the start of the single‐blind period (Week 0) to Week 8 and Week 12 (intent‐to‐treat population on randomized treatment). ***p<0.0001 vs monotherapy; †p<0.05 vs valsartan/hydrochlorothiazide (HCTZ) 320/12.5 mg. LSM, least squares means.
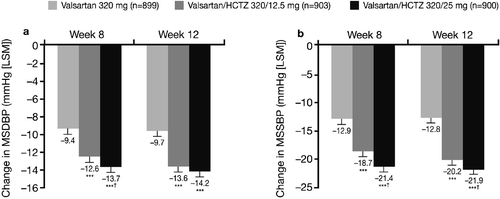
Figure 3. Proportion of responders at the end of the study(Week 12) (intent‐to‐treat population). ***p<0.0001 vs monotherapy; †p<0.05 vs valsartan/hydrochlorothiazide (HCTZ) 320/12.5 mg. Diastolic blood pressure (DBP) responders defined as patients with mean sitting DBP (MSDBP) <90 mmHg at endpoint and/or decrease in MSDBP of 10 mmHg from start of double‐blind period; systolic blood pressure (SBP) responders defined as patients with mean sitting SBP (MSSBP) <140 mmHg at endpoint and/or decrease in MSSBP of 20 mmHg from start of double‐blind period.
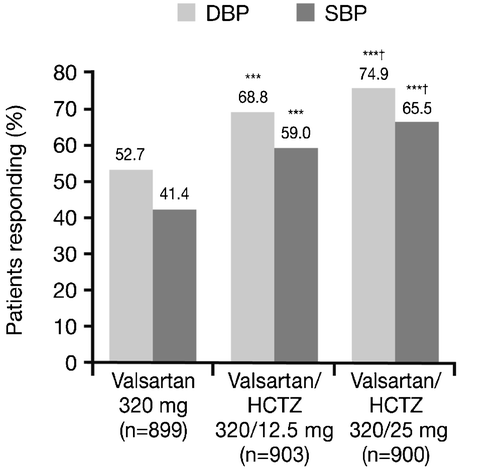
Figure 4. Proportion of patients with adequately controlled blood pressure(BP) at the end of the study (Week 12) (intent‐to‐treat population). ***p<0.0001 vs monotherapy; †p<0.05 vs valsartan/hydrochlorothiazide (HCTZ) 320/12.5 mg. Diastolic blood pressure (DBP) control defined as patients with mean sitting DBP (MSDBP) <90 mmHg from the start of the double‐blind period to study endpoint; systolic blood pressure (SBP) control defined as patients with mean sitting SBP (MSSBP) <140 mmHg from the start of the double‐blind period to study endpoint; overall control defined as MSSBP <140 mmHg and MSDBP <90 mmHg.
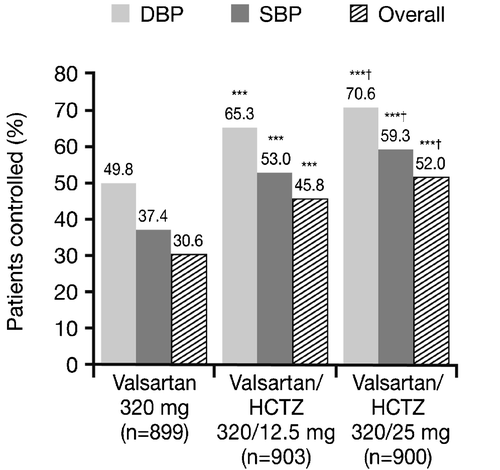
Figure 5. Mean sitting diastolic blood pressure(MSDBP) (a) and mean sitting systolic blood pressure (MSSBP) (b) changes from the start of the single‐blind period (Week 0) to Week 8 and Week 12 in ITT patients on randomized treatment with MSDBP ⩾100 mmHg after receiving the initial 4 weeks valsartan 320 mg monotherapy. ***p<0.0001 vs monotherapy; †p<0.05 vs valsartan/hydrochlorothiazide (HCTZ) 320/12.5 mg. LSM, least squares means.
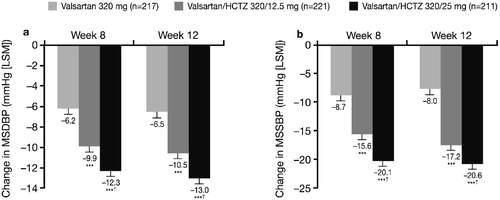
Table II. Incidence of most common adverse events (⩾1% in any treatment group) during the active treatment period (safety population).
Table III. Number (%) of patients with abnormal biochemistry values during the double‐blind treatment period (double‐blind safety population).