Figures & data
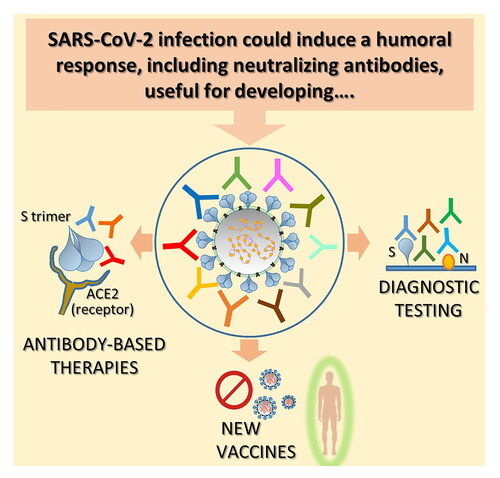
Figure 1. Humoral response against SARS-CoV-2 and its implication: key messages. A. SARS-CoV-2 is a single-stranded positive-sense RNA virus which infects human cells expressing ACE2 receptor through its trimeric surface spike (S). Glycoprotein Nucleocapsid (N) protein is a highly immunogenic structural protein which participate in RNA package and virus particle release. The most common observations regarding the humoral response mounted by SARS-CoV-2 infected patients are summarized. B. Different serologic tests have been promptly implemented for the diagnosis of SARS-CoV-2 infection diagnosis. At present, serum antibodies against S and N proteins are the ones mainly detected. Potentialities and drawbacks of this kind of assay are summarized. C. Antibody response against SARS-CoV-2 has been used for the implementation of convalescent plasma therapy (up) and the isolation and characterization of neutralizing mAbs for therapy (down). Salient points concerning these two treatment options are reported. D. Both analysis of neutralizing response against SARS-CoV-2 and epitope characterization of isolated monoclonal Nabs are useful for vaccine design. Focal points of the design of a vaccine aimed to induce an effective antibody response are highlighted.
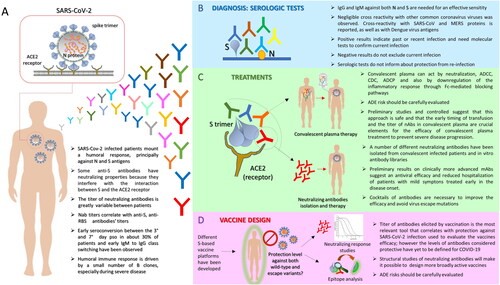
Figure 2. Antibody structure and function. A. Protective functions of antibodies are summarized. Antibodies are able to deploy a plethora of effector functions over the course of an infection: the antigen binding site of a neutralizing mAb is specifically directed against the viral surface antigen and directly interferes with virus-cell receptor interaction (on the top); human IgGs, particularly IgG1 and IgG3, bound to to the viral antigen exposed on the target cell, can subsequently interact through their Fc region with FcγRs expressed by effector cells or with complement component 1q, potentially supporting the destruction of target cells through ADCC or CDC, respectively. In addition, the Fc region of IgG can bind the salvage receptor FcRn after fluid-phase uptake by vascular endothelial cells and other cells, an interaction that contributes to the long (∼21 day) half-life of human IgG. B. The ADE phenomenon is illustrated as a possible adverse event that could occur with some antibodies. ADE has been documented to occur through two distinct mechanisms in viral infections: by enhanced antibody-mediated virus uptake into FcγR-expressing phagocytic cells leading to increased viral infection and replication, or by excessive antibody Fc-mediated effector functions or immune complex formation causing enhanced inflammation and immunopathology. Both ADE pathways can occur when non-neutralizing antibodies or antibodies at sub-neutralizing levels bind to viral antigens without blocking or clearing the infection. Identification and exclusion of ADE-associated epitopes in vaccine design or modification of the amino acid sequence of IgG reducing the interaction with one or more binding partners (for example by inserting LALA mutations) could be promising strategies to improve the clinical potential of antibodies.
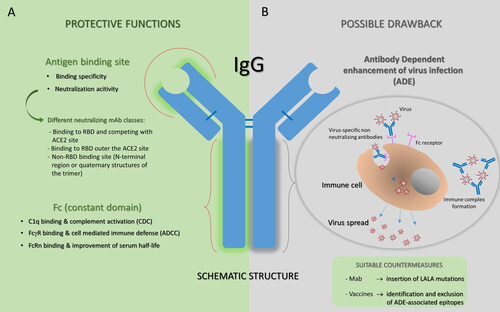
Table 1. Clinical studies on convalescent plasma therapy: characteristics and results.
Table 2. SARS-CoV cross-reactive monoclonal antibodies.
Table 3. SARS-CoV-2 specific monoclonal antibodies.
