Figures & data
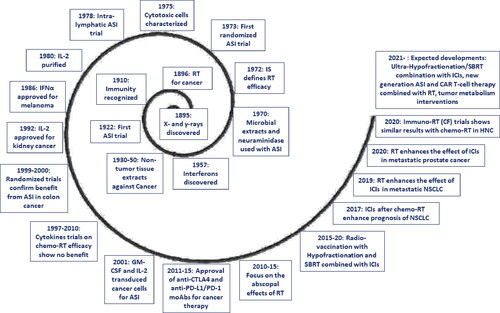
Figure 1. Schematic representation of the outcome of radiotherapy. Following the completion of radiotherapy or chemo-radiotherapy, tumors with high radioresistance will show no signs of regression. In a minority of cases, the tumor grows rapidly after (or during) radiotherapy. More radio-sensitive tumors will show signs of regression, even during radiotherapy, a process that continues for a couple of months. Generally, eradication of tumors occurs in about 70-90% of early and 5-40% of locally advanced stages. Persistent disease, either subclinically or clinically, enters a phase of short or long-lasting equilibrium between proliferation and death, senescence, quiescence, and stem-cell activity. The immune system plays an important role in the maintenance of this equilibrium. Overactivation may eradicate the tumor, while the development of immune tolerance (e.g., the emergence of cancer cell clones with immune checkpoint inhibitory molecule overexpression, the prevalence of regulatory immune cells that suppress cancer cell recognition of cytotoxic cells and the gradual built of an immunosuppressive microenvironment) will shift the balance toward tumor regrowth.
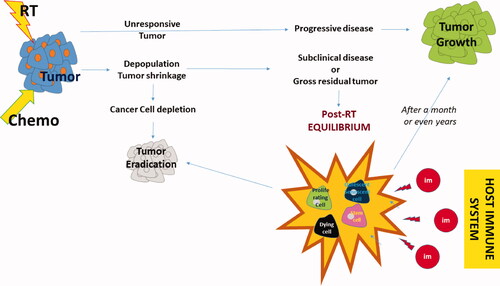
Table 1. Randomized trials of active specific immunotherapy.
Table 2. Randomized trials on combinations of radiotherapy with cytokines.
Table 3. Randomized trials on the combination of immune checkpoint inhibitors with RT.
Figure 2. The ‘circle’ of antitumor immune response: Tumor cells grow and dendritic cells have a poor ability to recognize them due to lack of antigenic presentation or inhibitory immune checkpoint molecule expression. RT damage induces radiovaccination, thus enhanced expression and release of tumor specific peptides, chemokine chemoatractive molecules and cytokines activating DCs and Tcells. Primed DCs carrying tumor antigens leave the tumor through peripheral lymphatic vessels and reach the RL. There these activate tumor specific Tcyt that leave the node through the central vein to reach the venous circulation. RT destroys DCs and Tcyt and the overall lymph node structure minimizing the efficacy of the radio-vaccination effect. Moreover, RT kills lymphocytes circulating in the normal tissues included in the RT fields, inducing strong lymphopenia and, further minimizing the radiovaccination effect. Extraction of autologous cancer cells, transduction to secrete cytokines and ex-vivo irradiation build a strong vaccine that can be injected in the efferent lymphatics of DLs, outside the RT portals. ASI reproduces the radiovaccination effect in undamaged lymph nodes that replace the radio-suppressed immune function of damaged RLs. Extraction of Tcells, activation with cytokines or development CAR-Tcells and re-infusion in the venous stream further boost the anti tumor immune cascade. ICIs or cytokines may shft the monocytic popolulation toward anti-tumor phenotypes that leave the bone-marrow to enrich the venous stream. Anti-tumor lymphocytes and monocytes reach metastatic sites to exert abscopal tumoricidal activity, controlling the tumor outside the RT portals. These cell will also reach the primary tumor shifting the post-RT equilibrium toward tumor eradication. ICIs further unmask cancer cells, block regulatory immune cells and allow killer cell activity. Cytokines that specifically prime cytotoxic activity and block regulatory activity may further assist the immunological elimination of the disease. (Green curved arrow: lymphatic circulation; red curved arrow: arterial circulation; blue curved arrow: venous circulation; green straight arrow: enhancement; red straight arrow: inhibition; green +: promotion; red X: blockage; RT = radiotherapy; RL = regional lymph node; DL = distal out-field lymph node; tT = transduction of Tcells; ASI = active specific immunotherapy; Tcyt = cytotoxic Tcell; DC = dendritic cell; LAK = lymphokine activated killer cells; CAR Tcell = chimeric antigen receptor Tcell; ICI = immune checkpoint inhibitors).
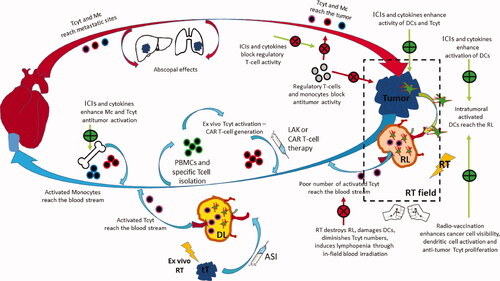
Figure 3. Key-stone events in the development of immuno-radiotherapy, during the long 125 years since the discovery of ionizing radiation (RT = radiotherapy; IS = immune system; ASI = active specific immunotherapy; moAB = monoclonal antibody; ICI = immune checkpoint inhibitors; NSCLC = non-small-cell lung cancer; CF = conventional fractionation; CAR-T = chimeric antigen receptor Tcell; SBRT = stereotactic body radiotherapy).