Figures & data
Table 1 Body weight, plasma and urine parameters in control and CRF rats
Figure 1. Differences in Na+ K+ ATPase activity and total Na+ K+ ATPase expression in outer renal medulla of control and CRF rats: (a) Na+ K+ ATPase activity was measured in outer renal medulla membranes by hydrolysis of [γ-32P]ATP; values are means ± SEM and expressed as μmol Pi / mg. prot . hr, and (b) Na+ K+ ATPase expression was measured in outer renal medulla homogenates using a common monoclonal antibody that recognizes total Na+ K+ ATPase α1-subunit independently of its phosphorylation degree; values are means ± SEM and were expressed as normalized arbitrary units. Normalized arbitrary units were obtained as total Na+ K+ ATPase α1-subunit expression with common antibody per μg protein. Na+ K+ ATPase activity and total Na+ K+ ATPase expression were increased in CRF rats as compared with control rats. *p < 0.05, as compared with control rats.
![Figure 1. Differences in Na+ K+ ATPase activity and total Na+ K+ ATPase expression in outer renal medulla of control and CRF rats: (a) Na+ K+ ATPase activity was measured in outer renal medulla membranes by hydrolysis of [γ-32P]ATP; values are means ± SEM and expressed as μmol Pi / mg. prot . hr, and (b) Na+ K+ ATPase expression was measured in outer renal medulla homogenates using a common monoclonal antibody that recognizes total Na+ K+ ATPase α1-subunit independently of its phosphorylation degree; values are means ± SEM and were expressed as normalized arbitrary units. Normalized arbitrary units were obtained as total Na+ K+ ATPase α1-subunit expression with common antibody per μg protein. Na+ K+ ATPase activity and total Na+ K+ ATPase expression were increased in CRF rats as compared with control rats. *p < 0.05, as compared with control rats.](/cms/asset/ce4dcd62-622e-46c0-8cad-6ad8a8117c79/irnf_a_203756_uf0001_b.gif)
Figure 2. Differences in α1-subunit Na+ K+ ATPase expression at Ser-23 in mTAL microdissected segments of control and CRF rats. The immunoblot was performed with a monoclonal antibody (McK-1) against dephosphorylated PKC site, Ser-23, of the Na+ K+ ATPase α1-subunit. Values are means ± SEM and were expressed as normalized arbitrary units. Normalized arbitrary units were obtained as (a) Na+ K+ ATPase α1-subunit expression with McK-1 antibody per μg protein, and (b) Na+ K+ ATPase α1-subunit expression with McK-1 antibody / total Na+ K+ ATPase α1-subunit expression with common antibody per μg protein in microdissected tubules. (see ). Densitometric analysis of all samples revealed a higher immunosignal (decreased phosphorylation) of Na+ K+ ATPase α1-subunit expression in CRF rats, as compared with control rats. *p < 0.05, as compared with control rats.
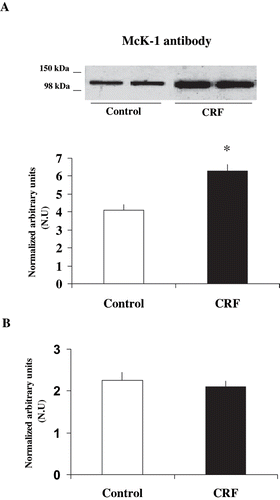
Table 2 Expression of α1-subunit Na+ K+ ATPase phosphorylation degree at Ser-23 in microdissected mTAL in control and CRF rats
Figure 3. Effect of PKC inhibition on α1-subunit Na+ K+ ATPase phosphorylation degree at Ser-23 in mTAL microdissected from control and CRF rats. Na+ K+ ATPase α1-subunit phosphorylation degree at Ser-23 in immunoblots of mTAL segments from control and CRF rats were treated with RO-318220 10−6 M, a specific PKC inhibitor. The immunoblot was performed with a monoclonal antibody (McK-1) against dephosphorylated PKC site, Ser-23, of the Na+ K+ ATPase α1-subunit. Values are means ± SEM and were expressed as percentage of normalized arbitrary units over basal. Normalized arbitrary units were obtained as Na+ K+ ATPase α1-subunit expression with McK-1 antibody / total Na+ K+ ATPase α1-subunit expression with common antibody per μg protein in microdissected tubules (see ). Densitometric analysis of all samples revealed an increase in immunosignal of Na+ K+ ATPase α1-subunit (decreased phosphorylation) under RO-318220 in mTAL segments of CRF rats. No changes were observed with RO-318220 in mTAL segments of control rats (p = NS). *p < 0.05, as compared with basal in CRF rats.
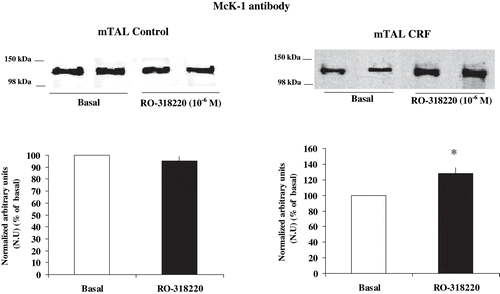
Figure 4. Effect of PKC stimulation on α1-subunit Na+ K+ ATPase phosphorylation degree at Ser-23 in mTAL microdissected segments from CRF rats. Na+ K+ ATPase α1-subunit phosphorylation degree at Ser-23 in immunoblots mTAL segments in CRF rats and under (a) phorbol 12-myristate 13-acetate (PMA) 10−6 M, a specific PKC agonist, or (b) dopamine (DA) 10−6 M. The immunoblot was performed with a monoclonal antibody (McK-1) against dephosphorylated PKC site, Ser-23, of the Na+ K+ ATPase α1-subunit. Values are means ± SEM and were expressed as percentage of normalized arbitrary units over basal. Normalized arbitrary units were obtained as Na+ K+ ATPase α1-subunit expression with McK-1 antibody / total Na+ K+ ATPase α1-subunit expression with common antibody per μg protein in microdissected tubule (see ). Densitometric analysis of all samples revealed a decrease in immunosignal (higher phosphorylation) of Na+ K+ ATPase α1-subunit under PMA and DA in mTAL segments of CRF rats. *p < 0.05, as compared with basal in CRF rats.
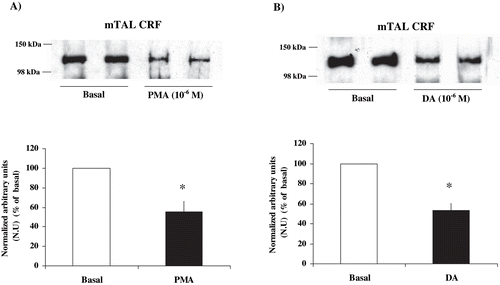
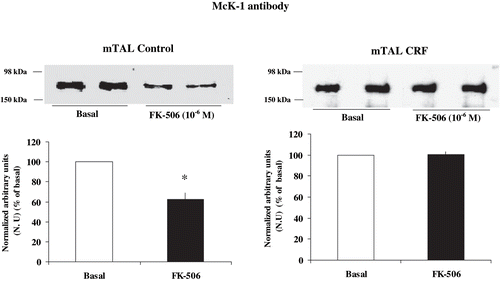
Figure 5. Effect of calcineurin inhibition on α1-subunit Na+ K+ ATPase phosphorylation degree at Ser-23 in mTAL microdissected segments of control and CRF rats. Na+ K+ ATPase α1-subunit phosphorylation degree at Ser-23 in immunoblots of mTAL segments from control and CRF rats were treated with FK-506 10−6 M, a specific calcineurin inhibitor. The immunoblot was performed with a monoclonal antibody (McK-1) against dephosphorylated PKC site, Ser-23, of the Na+ K+ ATPase α1-subunit. Values are means ± SEM and were expressed as percentage of normalized arbitrary units over basal. Normalized arbitrary units were obtained as Na+ K+ ATPase α1-subunit expression with McK-1 antibody / total Na+ K+ ATPase α1-subunit expression with common antibody per μg protein in microdissected tubules (see ). Densitometric analysis of all samples revealed no changes in immunosignal of α1-subunit Na+ K+ ATPase at Ser-23 under FK-506 in mTAL segments of CRF rats (p = NS). The calcineurin inhibitor FK-506 decreased immunosignal (higher phosphorylation) in mTAL of control rats. *p < 0.05, as compared with basal in control rats.