Figures & data
Figure 1. (A) Effect of various MGO concentrations (10 μM, 50 μM, 100 μM, 200 μM) on the viability of rat renal mesangial cells. (B) MGO increased caspase-3 activation of mesangial cells. (C) Inhibition of superoxide by SOD or DPI, suppression of P38 activity by SB203580, and inhibition of Ras activity manumycin A rescued the promoting effect of MGO on cleaved caspase 3 protein expression. Total extract of cell cultures were subjected to Western blot assays. The relative intensities of the immunoblotting bands were measured by densitometry normalized to each vehicle. Experimental results are presented as means ± standard errors calculated from three triplicate experiments.
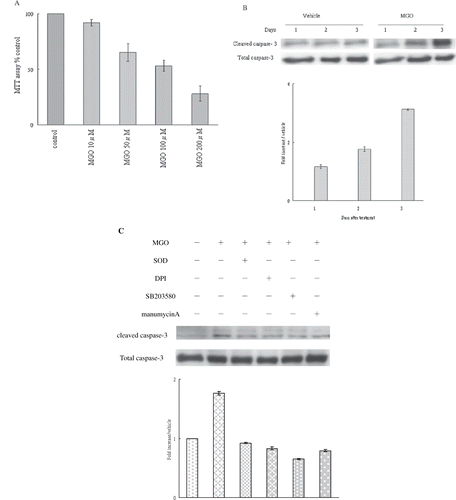
Figure 2. (A) MGO increased superoxide synthesis by mesangial cells in 2 hours to 24 hours. (B) Inhibition of superoxide by DPI and manumycin A, but not by rotenone, allopurinol, or SB203580, reduced MGO augmented superoxide production at 6 hour. Results are presented with mean values ± standard errors calculated from six paired triplicate experiments. * and # respectively show the difference from vehicle and MGO group, p < 0.05.
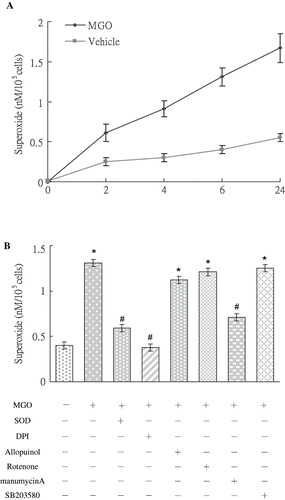
Figure 3. (A) MGO rapidly increased Ras activation in rat renal mesangial cells. (B) Inhibition of Ras activity by manumycin A but not inhibition of superoxide by SOD reduced the promoting effect of high MGO on Ras activation at 1 hour. Cell cultures (1 × 106 cells/dish, 100mm culture dish) were pretreated with 2.5μM manumycin A and subjected to high MGO treatment. The relative intensities of the immunoblotting bands were measured by densitometry normalized to each vehicle. Experimental results are presented as means ± standard errors calculated from three triplicate experiments.
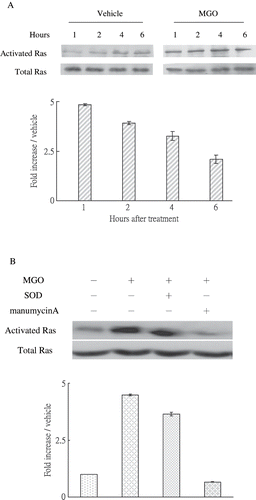
Figure 4. (A) High MGO increased cytosolic P38 activation. (B) Inhibition of superoxide by SOD or DPI or suppression of P38 activity by SB203580 and inhibition of Ras activity by manumycin A reduced the promoting effect of MGO on cytosolic P38 activation in four hours. Cytosolic fractions of cell lysate from cultures were subjected to Western blot assay. Immunoblotting of P38 showed equal loadings and transfer for all lanes. The relative intensities of the immunoblotting bands were measured by densitometry normalized to each vehicle. Experimental results are presented as means ± standard errors calculated from three triplicate experiments.
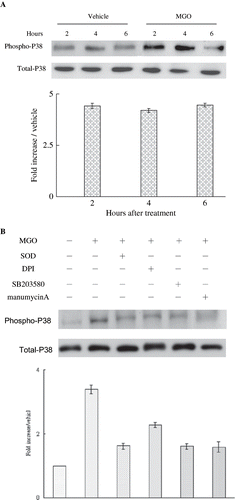
Figure 5. (A) MGO-induced nuclear c-Jun activation. Inhibition of superoxide by SOD or DPI, suppression of P38 activity by SB203580, and inhibiting Ras activity by manumycin A reduced the promoting effect of MGO on nuclear c-Jun activation. Cytosolic and nuclear fractions of cell lysate from cultures were subjected to Western blot assay. Immunoblotting of total c-Jun showed equal loadings and transfer for all lanes. The relative intensities of the immunoblotting bands were measured by densitometry normalized to each vehicle. Experimental results are presented as means ± standard errors calculated from three triplicate experiments.
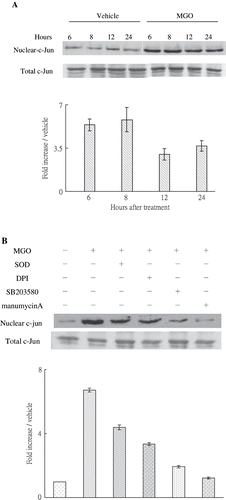
Figure 6. The profile in vehicle group (A) and the percentage of cells undergoing apoptosis (annexin V-positive, lower panel) under various conditions (B) were shown. Role of NADPH oxidase, P38 MAP kinase, and Ras signaling pathways was in MGO-induced mesangial cell apoptosis. SOD, DPI, SB203580, and manumycinA significantly reduced the percentage of apoptosis. The results shown represent the means ± SD of three experiments. * and # respectively show the difference from the vehicle and MGO group, p < 0.05.
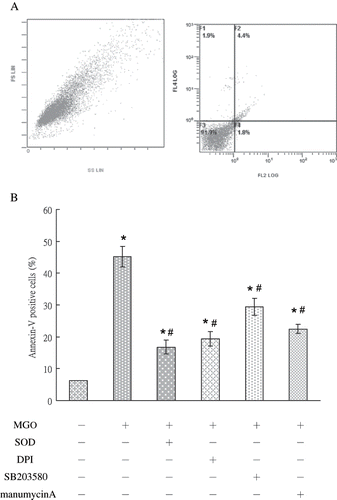
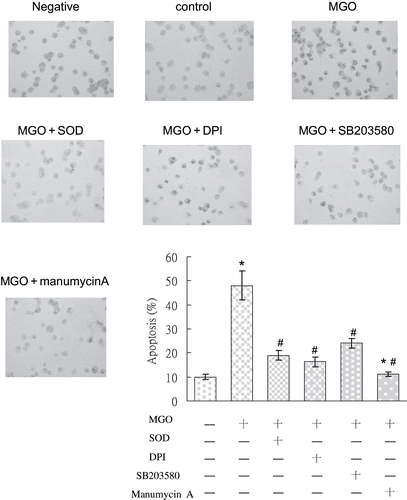
Figure 7. TUNEL staining photography of MGO-treated RMC with or without various treatments, including SOD, DPI, SB203580, and manumycin A. In comparison with the control group, mesangial cells in MGO treatment expressed strong TUNEL immunostaining. Decreased TUNEL staining was observed after pretreatment with SOD, DPI, SB203580, and manumycin A, individually. Specimens were observed in magnification = 200×.