Figures & data
Figure 1. The effects of LIPUS and SonoVue on proliferation, viability and apoptosis of HRGEC. (A) MTT assay of HRGECs treated with LIPUS, SonoVue, or LIPUS combined with SonoVue. The optical density reading was converted to relative cell number. n = 4. n.s., non-significant. *p < .05; (B) Trypan blue exclusion of assay of HRGECs treated with LIPUS, SonoVue, or LIPUS combined with SonoVue. n = 4. n.s., non-significant. **p < .01; (C) Representative images of flow cytometry analyses of Annexin V/PI double staining for HRGECs with treated with LIPUS, SonoVue, or LIPUS combined with SonoVue, following by incubation with fresh media for 24 h. (D) Analyses of the apoptosis rate obtained from Annexin V-FITC/PI assay. n = 4. n.s., non-significant. *p < .05; U: LIPUS; S: SonoVue; U + S: LIPUS and SonoVue.
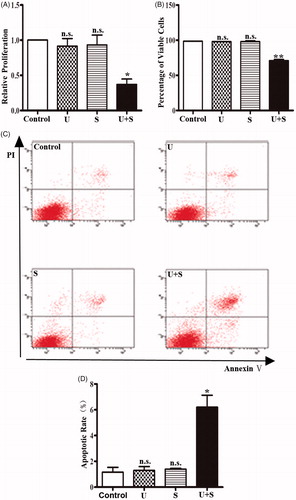
Figure 2. LIPUS in combination with SonoVue decreases phosphorylation of ERK1/2 in HRGECs. (A) Representative blots of phospho-ERK1/2 and total ERK1/2 from samples of HRGECs treated with LIPUS, SonoVue, or LIPUS combined with SonoVue. (B) Densitometry analyses of phospho-ERK1/2/total ERK1/2 of (A). n = 4. n.s., non-significant. **p < .01; (C) Representative blots of phosphor-ERK and total ERK from samples of HRGECs treated with LIPUS combined with SonoVue for 12 and 24 h after treatments. (D) Densitometry analyses of phospho-ERK1/2/total ERK1/2 of (A). n = 4, *p < .05; U: LIPUS; S: SonoVue; U + S: LIPUS; and SonoVue. GAPDH was used as loading control.
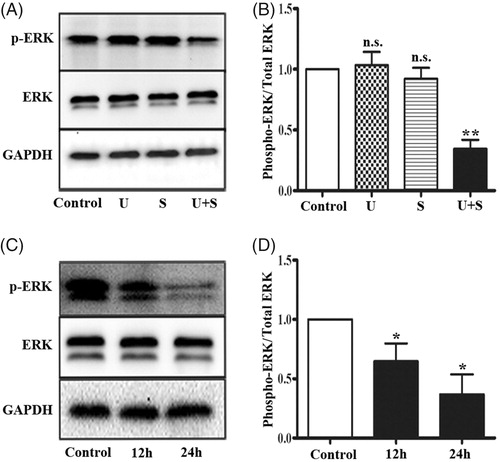
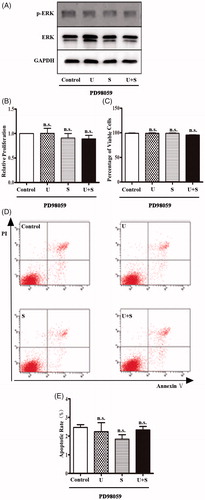
Figure 3. The effects of LIPUS and SonoVue on proliferation, viability, and apoptosis of HRGEC pretreated with PD98059. (A) Representative blots of phospho-ERK1/2 and total ERK1/2 from samples of HRGECs treated with LIPUS, SonoVue, or LIPUS combined with SonoVue after 1 h pretreatment of PD98059. GAPDH was used as loading control. (B) MTT assay of HRGECs treated with LIPUS, SonoVue, or LIPUS combined with SonoVue after 1 h pretreatment of PD98059. The optical density reading was converted to relative cell number. n = 4. n.s., non-significant; (C) Trypan blue exclusion of assay of HRGECs treated with LIPUS, SonoVue, or LIPUS combined with SonoVue after 1 h pretreatment of PD98059. n = 4. n.s., non-significant; (D) Representative images of flow cytometry analyses of Annexin V/PI double staining for HRGECs with treated with LIPUS, SonoVue, or LIPUS combined with SonoVue after 1 h pretreatment of PD98059, following by incubation with fresh media for 24 h. (E) Analyses of the apoptosis rate obtained from Annexin V-FITC/PI assay. n = 4. n.s., non-significant; U: LIPUS; S: SonoVue; U + S: LIPUS and SonoVue.