Figures & data
Table 1. Basic data in the two groups.
Figure 1. Clinical efficacy in the two groups. After treatment for 16 weeks and 24 weeks, the remission rates were similar between the two groups (p > .05). PR: partial remission; CR: complete remission.
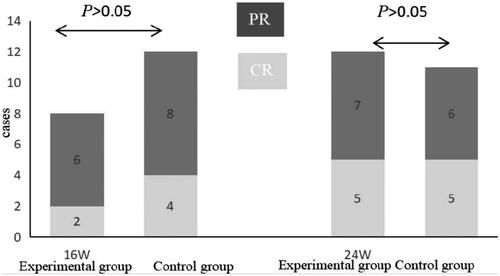
Figure 2. Albumin and 24-h urinary protein levels in the two groups. Before treatment, there was no significant difference in albumin or 24-h urinary protein levels between the two groups (#p > .05); after 16 weeks of treatment, there were significant differences in albumin or 24-h urinary protein levels between the two groups (##p < .05). 24-h UTP: 24-h urinary protein; ALB: albumin.
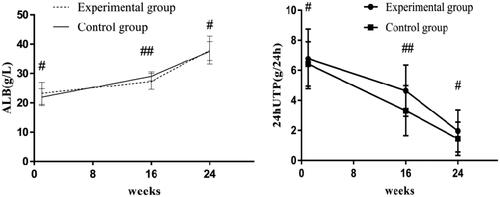
Figure 3. 24-h urinary protein and serum albumin levels and serum anti- PLA2R antibody titers. Serum anti-PLA2R antibody titers showed a positive correlation with 24-h urinary protein levels (p < .05) and a negative correlation with serum albumin levels (p < .05). 24hUTP: 24h urinary protein; ALB: serum albumin.
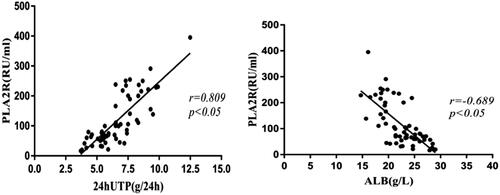
Table 2. Comparison of serum anti-PLA2R antibody titers between the two groups.
Figure 4. Serum anti-PLA2R antibody titers in patients with different prognoses. The serum PLA2R antibody titers in patients with complete remission and partial remission decreased significantly after treatment (p < .05), and there was no significant difference in the serum PLA2R antibody titers of patients with nonremission after treatment (p > .05). PR: partial remission; CR: complete remission; NR: nonremission.
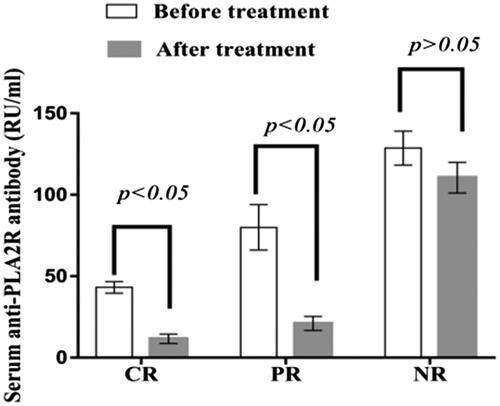
Table 3. Comparison of eGFR between the two groups.
Figure 5. Renal function before and after treatment in the two groups. Before and after treatment, there was no significant difference in Scr and BUN between the two groups (p > 0.05). Scr: serum creatinine; BUN: blood urea nitrogen.
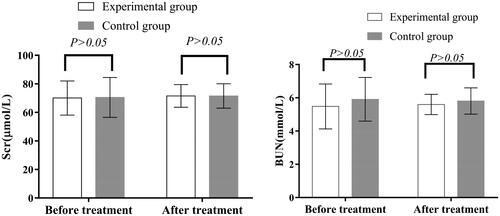
Table 4. Changes in blood lipids before and after treatment in the two groups.
