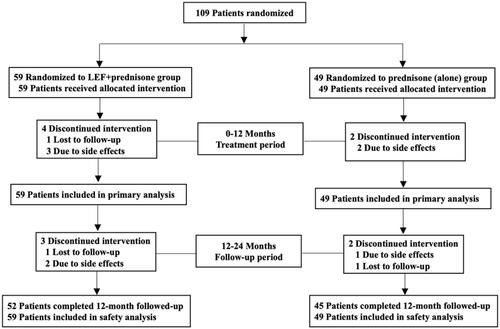
Figures & data
Table 1. Baseline characteristics.
Table 2. Outcomes of treatment.
Table 3. The complete, partial, and overall response between LEF + prednisone group and prednisone (alone) group.
Table 4. The relapsing rate between LEF + prednisone group and prednisone (alone) group.
Figure 2. The dosage of prednisone in the LEF + prednisone group was much lower than that in the prednisone (alone) group. ***p < 0.001 vs. prednisone (alone) group.
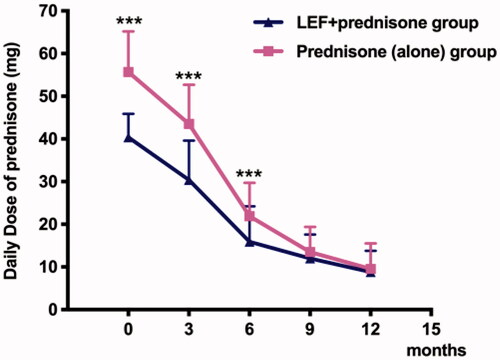
Table 5. The daily prednisone dose in LEF + prednisone group and prednisone (alone) group.
Table 6. Adverse events during the treatment period.
Data availability statement
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.